8.1: What is life history?
- Page ID
- 78352
Life history theory
Life history theory is an analytical framework[1] designed to study the diversity of life history strategies used by different organisms throughout the world, as well as the causes and results of the variation in their life cycles.[2] It is a theory of biological evolution that seeks to explain aspects of organisms' anatomy and behavior by reference to the way that their life histories—including their reproductive development and behaviors, post-reproductive behaviors, and life span (length of time alive)—have been shaped by natural selection. A life history strategy is the "age- and stage-specific patterns"[2] and timing of events that make up an organism's life, such as birth, weaning, maturation, death, etc.[3] These events, notably juvenile development, age of sexual maturity, first reproduction, number of offspring and level of parental investment, senescence and death, depend on the physical and ecological environment of the organism.
The theory was developed in the 1950s[4] and is used to answer questions about topics such as organism size, age of maturation, number of offspring, life span, and many others.[5] In order to study these topics, life history strategies must be identified, and then models are constructed to study their effects. Finally, predictions about the importance and role of the strategies are made,[6] and these predictions are used to understand how evolution affects the ordering and length of life history events in an organism's life, particularly the life span and period of reproduction.[7] Life history theory draws on an evolutionary foundation, and studies the effects of natural selection on organisms, both throughout their lifetime and across generations.[8] It also uses measures of evolutionary fitness to determine if organisms are able to maximize or optimize this fitness,[9] by allocating resources to a range of different demands throughout the organism's life.[1] It serves as a method to investigate further the "many layers of complexity of organisms and their worlds".[10]
Organisms have evolved a great variety of life histories, from Pacific salmon, which produce thousands of eggs at one time and then die, to human beings, who produce a few offspring over the course of decades. The theory depends on principles of evolutionary biology and ecology and is widely used in other areas of science.

Figure \(\PageIndex{1}\): A swallowtail butterfly hatches from a chrysalis. Image: Pixabay
Life cycle
All organisms follow a specific sequence in their development,[9] beginning with gestation and ending with death, which is known as the life cycle. Events in between usually include birth, childhood, maturation, reproduction, and senescence, and together these comprise the life history strategy of that organism.[3] The major events in this life cycle are usually shaped by the demographic qualities of the organism.[2] Some are more obvious shifts than others, and may be marked by physical changes—for example, teeth erupting in young children.[8] Some events may have little variation between individuals in a species, such as length of gestation, but other events may show a lot of variation between individuals,[3] such as age at first reproduction. Life cycles can be divided into two major stages: growth and reproduction. These two cannot take place at the same time, so once reproduction has begun, growth usually ends.[9] This shift is important because it can also affect other aspects of an organism's life, such as the organization of its group or its social interactions.[8]
Each species has its own pattern and timing for these events, often known as its ontogeny, and the variety produced by this is what life history theory addresses.[12] Evolution then works upon these stages to ensure that an organism adapts to its environment.[5] For example, a human, between being born and reaching adulthood, will pass through an assortment of life stages, which include: birth, infancy, weaning, childhood and growth, adolescence, sexual maturation, and reproduction.[3][12] All of these are defined in a specific biological way, which is not necessarily the same as the way that they are commonly used.[12] In life history theory, evolution works on the life stages of particular species (e.g., length of juvenile period) but is also discussed for a single organism's functional, lifetime adaptation. In both cases, researchers assume adaptation—processes that establish fitness.[5]
Traits
There are at least seven traits that are traditionally recognized as important in life history theory.[4] The trait that is seen as the most important for any given organism is the one where a change in that trait creates the most significant difference in that organism's level of fitness. In this sense, an organism's fitness is determined by its changing life history traits.[6] The way in which evolutionary forces act on these life history traits serves to limit the genetic variability and heritability of the life history strategies,[4] although there are still large varieties that exist in the world.
Commonly studied life history traits:
- size at birth
- growth pattern
- age and size at maturity
- number, size, and sex ratio of offspring
- age- and size-specific reproductive investments
- age- and size-specific mortality schedules
- length of life
Strategies
Combinations of these life history traits and life events create the life history strategies. As an example, Winemiller and Rose propose three types of life history strategies in the fish they study: opportunistic, periodic, and equilibrium.[13] These types of strategies are defined by the body size of the fish, age at maturation, high or low survivorship, and the type of environment they are found in. A fish with a large body size, a late age of maturation, and low survivorship, found in a seasonal environment, would be classified as having a periodic life strategy.[13] The type of behaviors taking place during life events can also define life history strategies. For example, an exploitative life history strategy would be one where an organism benefits by using more resources than others, or by taking these resources from other organisms.[14]
Ecological conditions favor organisms with certain life history strategies through natural selection and evolution, rather than organisms favoring certain strategies based on ecological conditions.
Characteristics
Life history characteristics are traits that affect the life table of an organism, and can be imagined as various investments in growth, reproduction, and survivorship. The goal of life history theory is to understand the variation in such life history strategies. This knowledge can be used to construct models to predict what kinds of traits will be favored in different environments. Without constraints, the highest fitness would belong to a Darwinian demon, a hypothetical organism for whom such trade-offs do not exist. The key to life history theory is that there are limited resources available, and focusing on only a few life history characteristics is necessary.
Examples of some major life history characteristics include:
- Age at first reproductive event
- Reproductive life span and aging
- Number and size of offspring
Variations in these characteristics reflect different allocations of an individual's resources (i.e., time, effort, and energy expenditure) to competing life functions. For any given individual, available resources in any particular environment are finite. Time, effort, and energy used for one purpose diminishes the time, effort, and energy available for another.
For example, birds with larger broods are unable to afford more prominent secondary sexual characteristics.[15] Life history characteristics will, in some cases, change according to the population density, since genotypes with the highest fitness at high population densities will not have the highest fitness at low population densities.[16] Other conditions, such as the stability of the environment, will lead to selection for certain life history traits. Experiments have found that unstable environments select for flies with both shorter life spans and higher fecundity—in unreliable conditions, it is better for an organism to breed early and abundantly than waste resources promoting its own survival.[17]
Trade-offs
An essential component of studying life history strategies is identifying the trade-offs[26] that take place for any given organism. Energy use in life history strategies is regulated by thermodynamics and the conservation of energy,[3] and the "inherent scarcity of resources",[9] so not all traits or tasks can be invested in at the same time. Thus, organisms must choose between tasks, such as growth, reproduction, and survival,[9] prioritizing some and not others. For example, there is a trade-off between maximizing body size and maximizing life span, and between maximizing offspring size and maximizing offspring number.[5][6] This is also sometimes seen as a choice between quantity and quality of offspring.[7] These choices are the trade-offs that life history theory studies.
One significant trade-off is between somatic effort (towards growth and maintenance of the body) and reproductive effort (towards producing offspring).[7][9] Since an organism cannot put energy towards doing these simultaneously, many organisms have a period where energy is put just toward growth, followed by a period where energy is focused on reproduction, creating a separation of the two in the life cycle.[3] Thus, the end of the period of growth marks the beginning of the period of reproduction. Another fundamental trade-off associated with reproduction is between mating effort and parenting effort. If an organism is focused on raising its offspring, it cannot devote that energy to pursuing a mate.[9]
An important trade-off in the dedication of resources to breeding has to do with predation risk: organisms that have to deal with an increased risk of predation often invest less in breeding. This is because it is not worth as much to invest a lot in breeding when the benefit of such investment is uncertain.[27]
These trade-offs, once identified, can then be put into models that estimate their effects on different life history strategies and answer questions about the selection pressures that exist on different life events.[7] Over time, there has been a shift in how these models are constructed. Instead of focusing on one trait and looking at how it changed, scientists are looking at these trade-offs as part of a larger system, with complex inputs and outcomes.[6]
The idea of constraints is closely linked to the idea of trade-offs discussed above. Because organisms have a finite amount of energy, the process of trade-offs acts as a natural limit on the organism's adaptations and potential for fitness. These limits can be physical, developmental, or historical, and they are imposed by the existing traits of the organism.[2]
Populations can adapt and thereby achieve an "optimal" life history strategy that allows the highest level of fitness possible (fitness maximization). There are several methods from which to approach the study of optimality, including energetic and demographic. Achieving optimal fitness also encompasses multiple generations, because the optimal use of energy includes both the parents and the offspring. For example, "optimal investment in offspring is where the decrease in total number of offspring is equaled by the increase of the number who survive".[7] Optimality is important for the study of life history theory because it serves as the basis for many of the models used, which work from the assumption that natural selection, as it works on a life history traits, is moving towards the most optimal group of traits and use of energy.[6] This base assumption, that over the course of its life span an organism is aiming for optimal energy use,[7] then allows scientists to test other predictions. However, actually gaining this optimal life history strategy cannot be guaranteed for any organism.[6]
An organism's allocation of resources ties into several other important concepts, such as trade-offs and optimality. The best possible allocation of resources is what allows an organism to achieve an optimal life history strategy and obtain the maximum level of fitness,[9] and making the best possible choices about how to allocate energy to various trade-offs contributes to this. The allocation of resources also plays a role in variation, because the different resource allocations by different species create the variety of life history strategies.[3]
Reproductive value and costs of reproduction
Reproductive value models the trade-offs between reproduction, growth, and survivorship. An organism's reproductive value (RV) is defined as its expected contribution to the population through both current and future reproduction:[19]
RV = Current Reproduction + Residual Reproductive Value (RRV)
The residual reproductive value represents an organism's future reproduction through its investment in growth and survivorship. The cost of reproduction hypothesis[20] predicts that higher investment in current reproduction hinders growth and survivorship and reduces future reproduction, while investments in growth will pay off with higher fecundity (number of offspring produced) and reproductive episodes in the future. This cost-of-reproduction trade-off influences major life history characteristics. For example, a 2009 study by Creighton et al. on burying beetles provided support for the costs of reproduction.[21] The study found that beetles that had allocated too many resources to current reproduction also had the shortest life spans. In their lifetimes, they also had the fewest reproductive events and offspring, reflecting how over-investment in current reproduction lowers residual reproductive value.
The related terminal investment hypothesis describes a shift to current reproduction with higher age. At early ages, RRV is typically high, and organisms should invest in growth to increase reproduction at a later age. As organisms age, this investment in growth gradually increases current reproduction. However, when an organism grows old and begins losing physiological function, mortality increases while fecundity decreases. This senescence shifts the reproduction trade-off towards current reproduction: the effects of aging and higher risk of death make current reproduction more favorable. The burying beetle study also supported the terminal investment hypothesis: the authors found beetles that bred later in life also had increased brood sizes, reflecting greater investment in those reproductive events.[22]
r/K selection theory
For more information on r/K selection see the Population Ecology chapter.
The selection pressures that determine the reproductive strategy, and therefore much of the life history, of an organism can be understood in terms of r/K selection theory. The central trade-off to life history theory is the number of offspring vs. the timing of reproduction. Organisms that are r-selected have a high growth rate (r) and tend to produce a high number of offspring with minimal parental care; their life spans also tend to be shorter. r-selected organisms are suited to life in an unstable environment, because they reproduce early and abundantly and allow for a low survival rate of offspring. K-selected organisms subsist near the carrying capacity of their environment (K), produce a relatively low number of offspring over a longer span of time, and have high parental investment. They are more suited to life in a stable environment in which they can rely on a long life span and a low mortality rate that will allow them to reproduce multiple times with a high offspring survival rate.[23]
Some organisms that are very r-selected are semelparous, only reproducing once before they die. Semelparous organisms may be short-lived, like annual crops. However, some semelparous organisms are relatively long-lived, such as the African flowering plant Lobelia telekii which spends up to several decades growing an inflorescence that blooms only once before the plant dies,[24] or the periodical cicada which spends 17 years as a larva before emerging as an adult. Organisms with longer life spans are usually iteroparous, reproducing more than once in a lifetime. However, iteroparous organisms can be more r-selected than K-selected, such as a sparrow, which gives birth to several chicks per year but lives only a few years, as compared to a wandering albatross, which first reproduces at ten years old and breeds every other year during its 40-year life span.[25]
r-selected organisms usually:
- mature rapidly and have an early age of first reproduction
- have a relatively short life span
- have a large number of offspring at a time, and few reproductive events, or are semelparous
- have a high mortality rate and a low offspring survival rate
- have minimal parental care/investment
K-selected organisms usually:
- mature more slowly and have a later age of first reproduction
- have a longer life span
- have few offspring at a time and more reproductive events spread out over a longer span of time
- have a low mortality rate and a high offspring survival rate
- have high parental investment

Figure \(\PageIndex{2}\): A litter of mice with their mother. The reproduction of mice follows an r-selection strategy, with many offspring, short gestation, less parental care, and a short time until sexual maturity Image: Seweryn Olkowicz
Unpredictable environments
Many factors can determine the evolution of an organism's life history, especially the unpredictability of the environment. A very unpredictable environment—one in which resources, hazards, and competitors may fluctuate rapidly—selects for organisms that produce more offspring earlier in their lives, because it is never certain whether they will survive to reproduce again. Mortality rate may be the best indicator of a species' life history: organisms with high mortality rates—the usual result of an unpredictable environment—typically mature earlier than those species with low mortality rates, and give birth to more offspring at a time.[32] A highly unpredictable environment can also lead to plasticity, in which individual organisms can shift along the spectrum of r-selected vs. K-selected life histories to suit the environment.[33]
Evolution Connection: Energy Budgets, Reproductive Costs, and Sexual Selection in Drosophila
Research into how animals allocate their energy resources for growth, maintenance, and reproduction has used a variety of experimental animal models. Some of this work has been done using the common fruit fly, Drosophila melanogaster. Studies have shown that not only does reproduction have a cost as far as how long male fruit flies live, but also fruit flies that have already mated several times have limited sperm remaining for reproduction. Fruit flies maximize their last chances at reproduction by selecting optimal mates.
In a 1981 study, male fruit flies were placed in enclosures with either virgin or inseminated females. The males that mated with virgin females had shorter life spans than those in contact with the same number of inseminated females with which they were unable to mate. This effect occurred regardless of how large (indicative of their age) the males were. Thus, males that did not mate lived longer, allowing them more opportunities to find mates in the future.
More recent studies, performed in 2006, show how males select the female with which they will mate and how this is affected by previous matings (Figure \(\PageIndex{3}\)). Males were allowed to select between smaller and larger females. Findings showed that larger females had greater fecundity, producing twice as many offspring per mating as the smaller females did. Males that had previously mated, and thus had lower supplies of sperm, were termed “resource-depleted,” while males that had not mated were termed “non-resource-depleted.” The study showed that although non-resource-depleted males preferentially mated with larger females, this selection of partners was more pronounced in the resource-depleted males. Thus, males with depleted sperm supplies, which were limited in the number of times that they could mate before they replenished their sperm supply, selected larger, more fecund females, thus maximizing their chances for offspring. This study was one of the first to show that the physiological state of the male affected its mating behavior in a way that clearly maximizes its use of limited reproductive resources.
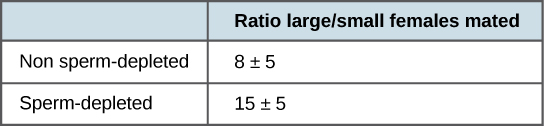
Figure \(\PageIndex{3}\): Male fruit flies that had previously mated (sperm-depleted) picked larger, more fecund females more often than those that had not mated (non-sperm-depleted). This change in behavior causes an increase in the efficiency of a limited reproductive resource: sperm.
These studies demonstrate two ways in which the energy budget is a factor in reproduction. First, energy expended on mating may reduce an animal’s life span, but by this time they have already reproduced, so in the context of natural selection this early death is not of much evolutionary importance. Second, when resources such as sperm (and the energy needed to replenish it) are low, an organism’s behavior can change to give them the best chance of passing their genes on to the next generation. These changes in behavior, so important to evolution, are studied in a discipline known as behavioral biology, or ethology, at the interface between population biology and psychology.
Adapted from Phillip G. Byrne and William R. Rice, “Evidence for adaptive male mate choice in the fruit fly Drosophila melanogaster,” Proc Biol Sci. 273, no. 1589 (2006): 917-922, doi: 10.1098/rspb.2005.3372.
Human life history
In studying humans, life history theory is used in many ways, including in biology, psychology, economics, anthropology, and other fields.[9][34][35] For humans, life history strategies include all the usual factors—trade-offs, constraints, reproductive effort, etc.—but also includes a culture factor that allows them to solve problems through cultural means in addition to through adaptation.[5] Humans also have unique traits that make them stand out from other organisms, such as a large brain, later maturity and age of first reproduction,[7] a long life span,[7][36] and a high level of reproduction, often supported by fathers and older (post-menopausal) relatives.[36][37][38] There are a variety of possible explanations for these unique traits. For example, a long juvenile period may have been adapted to support a period of learning the skills needed for successful hunting and foraging.[7][36] This period of learning may also explain the longer life span, as a longer amount of time over which to use those skills makes the period needed to acquire them worth it.[8][36] Cooperative breeding and the "grandmother hypothesis" have been proposed as the reasons that humans continue to live for many years after they are no longer capable of reproducing.[7][38] The large brain allows for a greater learning capacity, and the ability to engage in new behaviors and create new things.[7] The change in brain size may have been the result of a dietary shift—towards higher quality and difficult to obtain food sources[36]—or may have been driven by the social requirements of group living, which promoted sharing and provisioning.[8] Research has also indicated that humans may pursue different reproductive strategies.[39][40][41]
Survivorship Curves
Another tool used by population ecologists is a survivorship curve, which is a graph of the number of individuals surviving at each age interval plotted versus time (usually with data compiled from a life table). These curves allow us to compare the life histories of different populations (Figure \(\PageIndex{4}\)). Humans and most primates exhibit a Type I survivorship curve because a high percentage of offspring survive their early and middle years—death occurs predominantly in older individuals. These types of species usually have small numbers of offspring at one time, and they give a high amount of parental care to them to ensure their survival. Birds are an example of an intermediate or Type II survivorship curve because birds die more or less equally at each age interval. These organisms also may have relatively few offspring and provide significant parental care. Trees, marine invertebrates, and most fishes exhibit a Type III survivorship curve because very few of these organisms survive their younger years; however, those that make it to an old age are more likely to survive for a relatively long period of time. Organisms in this category usually have a very large number of offspring, but once they are born, little parental care is provided. Thus these offspring are “on their own” and vulnerable to predation, but their sheer numbers assure the survival of enough individuals to perpetuate the species.
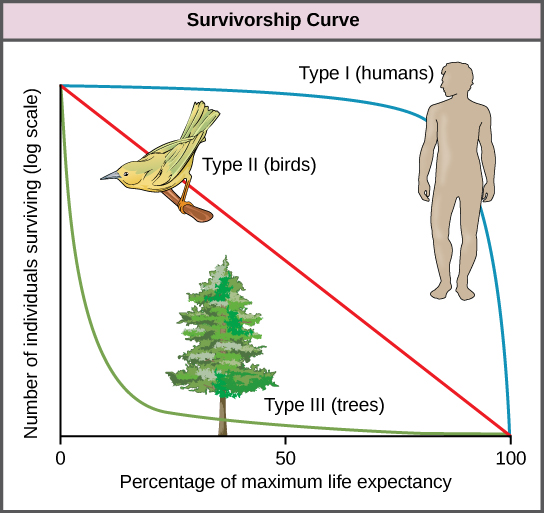
Figure \(\PageIndex{4}\): Survivorship curves show the distribution of individuals in a population according to age. Humans and most mammals have a Type I survivorship curve because death primarily occurs in the older years. Birds have a Type II survivorship curve, as death at any age is equally probable. Trees have a Type III survivorship curve because very few survive the younger years, but after a certain age, individuals are much more likely to survive.
Sources
- Vitzthum, V. (2008). Evolutionary models of women's reproductive functioning. Annual Review of Anthropology, 37, 53-73
- Flatt, T., & Heyland, A. (Eds.). (2011). Mechanisms of Life History Evolution : The Genetics and Physiology of Life History Traits and Trade-Offs. Oxford, GB: OUP Oxford.
- Ahlström, T. (2011). Life‐history theory, past human populations and climatic perturbations. International Journal of Osteoarchaeology, 21(4), 407-419.
- Stearns, S. (1992). The Evolution of Life Histories. Oxford ; New York: Oxford University Press.
- Hochberg, Z. (2011). Evo-Devo of Child Growth : Treatise on Child Growth and Human Evolution (1). Hoboken, US: Wiley-Blackwell.
- Stearns, S. (1976). Life-History Tactics: A Review of the Ideas. The Quarterly Review of Biology, 51(1), 3-47. JSTOR 2825234
- Hill, K., & Kaplan, H. (1999). Life history traits in humans: Theory and empirical studies. Annual Review Of Anthropology, 28(1), 397.
- Bolger, D. (Ed.). (2012). Wiley Blackwell Companions to Anthropology Ser. : A Companion to Gender Prehistory (1). Somerset, US: Wiley-Blackwell.
- Preston, S. D., Kringelbach, M. L., & Knutson, B. (2014). The Interdisciplinary Science of Consumption. Cambridge, US: The MIT Press.
- Morbeck, M., Galloway, A., & Zihlman, A. The Evolving Female : A Life-history Perspective. (1997). Princeton, N.J.: Princeton University Press
- Roff, D. (2002). Life History Evolution. Sunderland, Mass.: Sinauer.
- Hawkes K., ed. The Evolution of Human Life History. (2006). Santa Fe : Oxford: School of American Research ; James Currey. Gen ed.
- Lartillot, N., & Delsuc, F. (2012). "Joint reconstruction of divergence times and life-history evolution in placental mammals using a phylogenetic covariance model". Evolution, 66(6), 1773-1787.JSTOR 41503481
- Reynolds, J., & McCrea, S. (2016). Life history theory and exploitative strategies. Evolutionary Psychology, 14(3),
- Gustafsson, L., Qvarnström, A., and Sheldon, B.C. 1995. Trade-offs between life-history traits and a secondary sexual character in male collared flycatchers. Nature 375, 311—313
- Mueller, L.D., Guo, P., and Ayala, F.J. 1991. Density dependent natural selection and trade-offs in life history traits. Science, 253: 433-435.
- Rose, M. and Charlesworth, B. A Test of Evolutionary Theories of Senescence. 1980. Nature 287, 141-142
- Keen, E. C. (2014). "trade-offs in bacteriophage life histories". Bacteriophage. 4 (1): e28365. doi:10.4161/bact.28365. PMC 3942329. PMID 24616839.
- Fisher, R. A. 1930. The genetical theory of natural selection. Oxford University Press, Oxford.
- Jasienska, Grazyna (2009-07-01). "Reproduction and life span: Trade-offs, overall energy budgets, intergenerational costs, and costs neglected by research". American Journal of Human Biology. 21 (4): 524–532. doi:10.1002/ajhb.20931. ISSN 1520-6300. PMID 19367577. S2CID 11440141.
- J. Curtis Creighton, Nicholas D. Heflin, and Mark C. Belk. 2009. Cost of Reproduction, Resource Quality, and Terminal Investment in a Burying Beetle. The American Naturalist, 174:673–684.
- J. Curtis Creighton, Nicholas D. Heflin, and Mark C. Belk. 2009. Cost of Reproduction, Resource Quality, and Terminal Investment in a Burying Beetle. The American Naturalist, 174:673–684.
- Stearns, S.C. 1977. The Evolution of Life History Traits: A Critique of the Theory and a Review of the Data. Annual Review of Ecology and Systematics, 8: 145-171
- Young, Truman P. 1984. The Comparative Demography of Semelparous Lobelia Telekii and Iteroparous Lobelia Keniensis on Mount Kenya. Journal of Ecology, 72: 637–650
- Ricklefs, Robert E. 1977. On the Evolution of Reproductive Strategies in Birds: Reproductive Effort. The American Naturalist, 111: 453–478.
- "105_2013_12_05_Trade-offs_1". idea.ucr.edu.
- Dillon, Kristen G; Conway, Courtney J; Skelhorn, John (2018). "Nest predation risk explains variation in avian clutch size". Behavioral Ecology. 29 (2): 301–311. doi:10.1093/beheco/arx130. ISSN 1045-2249.
- Houston, Alasdair I.; Stephens, Philip A.; Boyd, Ian L.; Harding, Karin C.; McNamara, John M. (2007). "Capital or income breeding? A theoretical model of female reproductive strategies". Behavioral Ecology. 18 (1): 241–250. doi:10.1093/beheco/arl080. ISSN 1465-7279.
- Drent, R. H.; Daan, S. (1980). "The prudent parent: energetic adjustments in avian breeding". Ardea. 38–90: 225–252. doi:10.5253/arde.v68.p225. ISSN 0373-2266.
- Ejsmond, Maciej Jan; Varpe, Øystein; Czarnoleski, Marcin; Kozłowski, Jan (2015). "Seasonality in offspring value and trade-offs with growth explain capital breeding". The American Naturalist. 186 (5): E111–E125. doi:10.1086/683119. ISSN 0003-0147. S2CID 87515085.
- Sainmont, Julie; Andersen, Ken H.; Varpe, Øystein; Visser, André W. (2014). "Capital versus income breeding in a seasonal environment". The American Naturalist. 184 (4): 466–476. doi:10.1086/677926. ISSN 0003-0147. PMID 25226182. S2CID 28848120.
- Promislow, D.E.L. and P.H. Harvey. 1990. Living fast and dying young: A comparative analysis of life-history variation among mammals. Journal of Zoology, 220:417-437.
- Baird, D. G., L. R. Linton and Ronald W. Davies. 1986. Life-History Evolution and Post-Reproductive Mortality Risk. Journal of Animal Ecology 55: 295-302.
- Mittal, C., Griskevicius, V., Simpson, J., & Kawakami, K. (2014). Sense of control under uncertainty depends on people's childhood environment: A life history theory approach. Journal of Personality and Social Psychology, 107(4), 621-637.
- Schmitt, D., & Rhode, P. (2013). The human polygyny index and its ecological correlates: Testing sexual selection and life history theory at the cross‐national level. Social Science Quarterly, 94(4), 1159-1184.
- Kaplan, H., Hill, K., Lancaster, J. and Hurtado, A. M. (2000), A theory of human life history evolution: Diet, intelligence, and longevity. Evol. Anthropol., 9: 156–185. doi:10.1002/1520-6505(2000)9:4<156::AID-EVAN5>3.0.CO;2-7
- Barton, R., Capellini, I., & Stevens, C. (2011). Maternal investment life histories, and the costs of brain growth in mammals. Proceedings of the National Academy of Sciences of the United States of America, 108(15), 6169-6174. JSTOR 41126625
- Isler, K., & van Schaik, C. (2012). Allomaternal care, life history and brain size evolution in mammals. Journal of Human Evolution, 63(1), 52-63.
- Kim, Yuri, and James J. Lee. "The genetics of human fertility." Current opinion in psychology 27 (2019): 41-45.
- Yao, Shuyang, Niklas Långström, Hans Temrin, and Hasse Walum. "Criminal offending as part of an alternative reproductive strategy: Investigating evolutionary hypotheses using Swedish total population data." Evolution and Human Behavior 35, no. 6 (2014): 481-488.
- Vall, Gemma, Fernando Gutiérrez, Josep M. Peri, Miguel Gárriz, Eva Baillés, Juan Miguel Garrido, and Jordi E. Obiols. "Seven dimensions of personality pathology are under sexual selection in modern Spain." Evolution and Human Behavior 37, no. 3 (2016): 169-178.
Contributors and Attributions
Modified by Dan Wetzel (University of Pittsburgh) from the following sources:
- Wikipedia: https://en.wikipedia.org/wiki/Life_history_theory
- Connie Rye (East Mississippi Community College), Robert Wise (University of Wisconsin, Oshkosh), Vladimir Jurukovski (Suffolk County Community College), Jean DeSaix (University of North Carolina at Chapel Hill), Jung Choi (Georgia Institute of Technology), Yael Avissar (Rhode Island College) among other contributing authors. Original content by OpenStax (CC BY 4.0; Download for free at http://cnx.org/contents/185cbf87-c72...f21b5eabd@9.87).

