12.1: Introduction
- Page ID
- 105841
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
Introduction
All organisms must break down organic molecules to release chemical energy to synthesize adenosine triphosphate (ATP). The energy stored in ATP can be released to perform cellular work. Organisms break down organic molecules, such as glucose, through the common processes of cellular respiration and fermentation (Figure 2). Cellular respiration is generally described as an aerobic process, requiring oxygen, which yields the most possible ATP generated from one molecule of glucose. But, technically, cellular respiration can occur in an anaerobic environment in some microorganisms. Anaerobic cellular respiration yields variable amounts of ATP, but much less than is generated in aerobic cellular respiration. In this laboratory, our discussion of cellular respiration will focus on aerobic cellular respiration.
Both aerobic cellular respiration and fermentation involve many chemical reactions that release high energy electrons from organic molecules and transfer the electrons to other molecules, often referred to as electron carriers (or coenzymes). These chemical reactions involving the transfer of electrons are called reduction-oxidation reactions, or redox reactions. In a redox reaction, one of the molecules gains electrons and becomes reduced (rig, reduction is gain of electrons) and one of the molecules loses electrons and becomes oxidized (oil, oxidation is loss of electrons). In cellular respiration, electrons are often transferred to the electron carrier nicotinamide adenine dinucleotide (NAD+). When this redox reaction occurs, the organic molecule that loses the electrons has been oxidized. When NAD+ gains the electrons it forms NADH. NADH is the reduced form of NAD.
\[\ce{NAD^{+} + 2e^{-} + H^{+} ->[\text{Redox Reaction}] NADH}\]
Aerobic cellular respiration involves a series of three processes of enzymatic chemical reactions: glycolysis, the citric acid cycle (also known as the Kreb’s cycle), and the electron transport chain. Aerobic cellular respiration begins in the cytoplasm with glycolysis and ends in the mitochondria with the citric acid cycle and the electron transport chain. Aerobic cellular respiration results in fully oxidizing glucose, and can yield a maximum of 32 ATP per glucose molecule. At the culmination of the electron transport chain, the electrons are passed to oxygen, a highly electronegative element, to form water. Therefore, at the end of this process, the high energy electrons that were previously a part of glucose are now at a lower energy state, as they are held very closely by the electronegative oxygen.
Fermentation is an anaerobic process of breaking down organic molecules. It occurs in the absence of oxygen. Fermentation breaks down organic molecules, such as glucose, into smaller organic molecule end products. Fermentation begins with the process of glycolysis to produce pyruvic acid and 2 net ATP. Enzymes then carry out chemical reactions to convert pyruvic acid into various fermentation end products. Two common types of fermentation are named for their end products, alcoholic fermentation and lactic acid fermentation. Fermentation produces organic end products that still contain high-energy electrons. Fermentation does not fully oxidize glucose, and yields only 2 net molecules of ATP, along with organic end products.
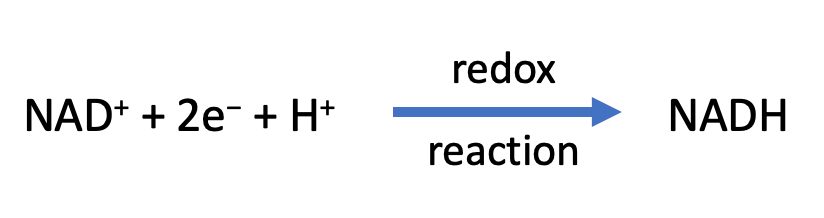 ( Figure \(\PageIndex{1}\): Redox reaction that occurs during cellular respiration)
( Figure \(\PageIndex{1}\): Redox reaction that occurs during cellular respiration)

