2.9: Energy in Chemical Reactions
- Last updated
- Save as PDF
- Page ID
- 30614
Slow Burn
These old iron chains give off a small amount of heat as they rust. The rusting of iron is a chemical process. It occurs when iron and oxygen go through a chemical reaction similar to burning, or combustion. The chemical reaction that occurs when something burns obviously gives off energy. You can feel the heat, and you may be able to see the light of flames. The rusting of iron is a much slower process, but it still gives off energy. It's just that it releases energy so slowly you can't detect a change in temperature.

What Is a Chemical Reaction?
A chemical reaction is a process that changes some chemical substances into others. A substance that starts a chemical reaction is called a reactant, and a substance that forms as a result of a chemical reaction is called a product. During the reaction, the reactants are used up to create the products.
Another example of a chemical reaction is the burning of methane gas, shown in Figure \(\PageIndex{2}\). In this chemical reaction, the reactants are methane (CH4) and oxygen (O2), and the products are carbon dioxide (CO2) and water (H2O). As this example shows, a chemical reaction involves the breaking and forming of chemical bonds. Chemical bonds are forces that hold together the atoms of a molecule. Bonds occur when atoms share electrons. When methane burns, for example, bonds break within the methane and oxygen molecules, and new bonds form in the molecules of carbon dioxide and water.

Chemical Equations
Chemical reactions can be represented by chemical equations. A chemical equation is a symbolic way of showing what happens during a chemical reaction. For example, the burning of methane can be represented by the chemical equation:
\[\ce{CH_4 + 2O_2 \rightarrow CO_2 + 2 H_2O}\]
The arrow in a chemical equation separates the reactants from the products and shows the direction in which the reaction proceeds. If the reaction could occur in the opposite direction as well, two arrows pointing in opposite directions would be used. The number 2 in front of O2 and H2O shows that two oxygen molecules and two water molecules are involved in the reaction. If just one molecule is involved, no number is placed in front of the chemical symbol.
Role of Energy in Chemical Reactions
Matter rusting or burning are common examples of chemical changes. Chemical changes involve chemical reactions, in which some substances, called reactants, change at the molecular level to form new substances, called products. All chemical reactions involve energy. However, not all chemical reactions release energy, as rusting and burning do. In some chemical reactions, energy is absorbed rather than released.
Exergonic Reactions
A chemical reaction that releases energy is called an exergonic reaction. This type of reaction can be represented by a general chemical equation:
\[\mathrm{Reactants \rightarrow Products + Energy}\]
Besides rusting and burning, examples of exothermic reactions include chlorine combining with sodium to form table salt. The decomposition of organic matter also releases energy because of exergonic reactions. Sometimes on a chilly morning, you can see steam rising from a compost pile because of these chemical reactions (see Figure \(\PageIndex{3}\)). Exergonic chemical reactions also take place in the cells of living things. In a chemical process similar to combustion, called cellular respiration, the sugar glucose is "burned" to provide cells with energy.

Endergonic Reactions
A chemical reaction that absorbs energy is called an endergonic reaction. This type of reaction can also be represented by a general chemical equation:
\[\mathrm{Reactants + Energy \rightarrow Products}\]
Did you ever use a chemical cold pack like the one in the picture below? The pack cools down because of an endergonic reaction. When a tube inside the pack is broken, it releases a chemical that reacts with water inside the pack. This reaction absorbs heat energy and quickly cools down the contents of the pack.

Many other chemical processes involve endergonic reactions. For example, most cooking and baking involves the use of energy to produce chemical reactions. You can't bake a cake or cook an egg without adding heat energy. Arguably, the most important endergonic reactions occur during photosynthesis. When plants produce sugar by photosynthesis, they take in light energy to power the necessary endergonic reactions. The sugar they produce provides plants and virtually all other living things with glucose for cellular respiration.
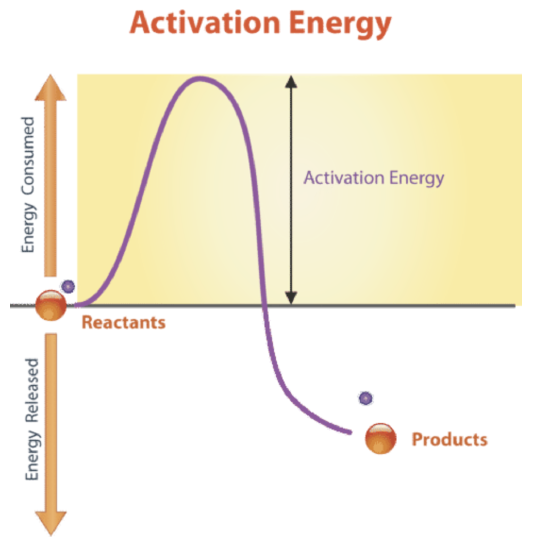
Activation Energy
All chemical reactions need energy to get started. Even reactions that release energy need a boost of energy in order to begin. The energy needed to start a chemical reaction is called activation energy. Activation energy is like the push a child needs to start going down a playground slide. The push gives the child enough energy to start moving, but once she starts, she keeps moving without being pushed again. Activation energy is illustrated in Figure \(\PageIndex{5}\).
Why do all chemical reactions need energy to get started? In order for reactions to begin, reactant molecules must bump into each other, so they must be moving, and movement requires energy. When reactant molecules bump together, they may repel each other because of intermolecular forces pushing them apart. Overcoming these forces so the molecules can come together and react also takes energy.
Review
- What is a chemical reaction?
- Identify reactants and products in a chemical reaction.
- List three examples of common changes that involve chemical reactions.
- Define a chemical bond.
- What is a chemical equation? Give an example.
- Our cells use glucose (C6H12O6) to obtain energy in a chemical reaction called cellular respiration. In this reaction, six oxygen molecules (O2) react with one glucose molecule. Answer the following questions about this reaction.
- How many oxygen atoms are in one molecule of glucose?
- Write out what the reactant side of this equation would look like.
- How many oxygen atoms are in the reactants in total? Explain how you calculated your answer.
- How many oxygen atoms are in the products in total? Is it possible to answer this question without knowing what the products are? Why or why not?
- Answer the following questions about the equation you saw above: CH4+ 2O2 → CO2 + 2H2O
- Can carbon dioxide (CO2) become transformed into methane (CH4) and oxygen (O2) in this reaction? Why or why not?
- How many molecules of carbon dioxide (CO2) are produced in this reaction?
- Is the evaporation of liquid water into water vapor a chemical reaction? Why or why not
- Why do bonds break in the reactants during a chemical reaction?
- Contrast endergonic and exergonic chemical reactions. Give an example of each.
- Define activation energy.
- Explain why all chemical reactions require activation energy.
- Heat is a form of ____________ .
- In which type of reaction is heat added to the reactants?
- In which type of reaction is heat produced?
- If there was no heat energy added to an endothermic reaction, would that reaction occur? Why or why not?
- If there was no heat energy added to an exothermic reaction, would that reaction occur? Why or why not?
- Explain why a chemical cold pack feels cold when activated.
- Explain why cellular respiration and photosynthesis are “opposites” of each other.
- Explain how the sun indirectly gives our cells energy.
Explore More
Watch the video below to learn more about activation energy.
Attributions
- Chaîne by Daplaza, licensed CC BY-SA 3.0 via Wikimedia Commons
- Gas Stove Burner Blue Flame by Federico Cardoner, licensed CC BY 2.0 via Flickr
- Compost steaming by Lucabon, CC BY-SA 4.0 via Wikimedia Commons
- Cooler pack by Julie Magro, licensed CC BY 2.0 via Flickr
- Activation energy by Hana Zavadska for CK-12 licensed CC BY-NC 3.0
- Text adapted from Human Biology by CK-12 licensed CC BY-NC 3.0


