2.5.14.11: Bioconversion Processes for Synthetic Chemicals
- Page ID
- 108931
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Most of the biochemical operations described so far in this chapter pertain to natural products which, by their nature, would be expected to be amenable to the action of enzymes. The mild conditions under which enzymes operate, the readily available, safe reagents that they employ, such as molecular O2 for oxidations, and the high specificity of enzyme catalysts make biocatalyzed reactions attractive for carrying out chemical processes on synthetic chemicals, such as those from petroleum sources. This section discusses two examples of enzyme-catalyzed processes applied to chemical processes on synthetic chemicals that would otherwise have to be performed with chemical reagents under much more severe conditions.
p-Hydroxybenzoic Acid from Toluene
The potential for use of biosynthesis applied to synthetic chemicals can be illustrated by the synthesis of p-hydroxybenzoic acid

an important intermediate used in the synthesis of pharmaceuticals, pesticides, dyes, preservatives, and liquid crystal polymers currently made by reacting potassium phenolate,

with carbon dioxide under high pressure at 220 ̊C, which converts slightly less than half of the potassium phenolate to the desired product and produces substantial impurities. The process dates back to the early 1860s almost 150 years ago, long before there were any considerations of pollutants and wastes. It requires severe conditions and produces metal and phenol wastes. Reactive alumina powder (Al2O3) used to catalyze the process has been implicated in a 1995 explosion at a facility to produce p-hydroxybenzoic acid that killed 4 workers.
A biosynthetic alternative to the synthesis described above has been attempted with Pseudomonasputida bacteria genetically engineered to carry out several steps in the synthesis of p-hydroxybenzoic acid starting with toluene. A key to the process is the attachment at the para position on toluene of a hydroxyl group by the action of toluene-4-monooxygenase (T4MO) enzyme system transferred to Pseudomonasputida from Pseudomonas mendocina:

The next step is carried out by p-cresol methylhydroxylase (PCMH) enzyme from a strain of Pseudomonasputida that yields p-hydroxybenzyl alcohol followed by conversion to p-hydroxybenzaldehyde:
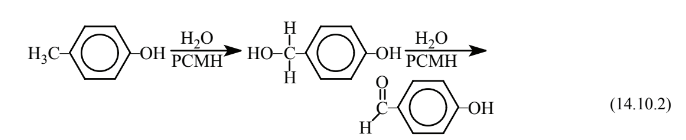
The last step is carried out by an aromatic aldehyde dehydrogenase enzyme designated PHBZ also obtained from a strain of Pseudomonas putida and consists of the conversion of the aldehyde to the p-hydroxybenzoic acid product:

Through elegant genetic manipulation, the chemical processes described above were achieved leading to the desired product. In addition to providing the enzymes to carry out the desired steps, it was also crucial to block steps that would consume intermediates and give undesired byproducts that would consume raw material and require separation from the product. Although it is a long way from showing that the complex biochemical synthesis process actually gives the desired product to the final goal of having a practical process that can be used on a large scale, the results described above certainly show the promise of transgenic organisms in carrying out chemical syntheses.
Production of 5-Cyanovaleramide
The second biocatalyzed process to be considered is the conversion of adiponitrile to 5-cyanovaleramide. This conversion was required for the synthesis of a new chemical used for crop protection. This process can be carried out chemically with a stochichiometric mixture of adiponitrile with water and a manganese dioxide catalyst under pressure at 130 ̊C as shown by the following reaction:

If the reaction is run to 25% completion, an 80% selectivity for the 5-cyanovaleramide is achieved, with the other fraction of the adiponitrile that reacts going to adipamide, in which the second C≡N functional group is converted to an amide group. Carrying the reaction beyond 25%completion resulted in unacceptable levels of conversion to byproduct adipamide.
The isolation of the 5-cyanovaleramide product from the chemical synthesis described above entails dissolving the hot reaction mixture in toluene solvent, which is then cooled to precipitate the product. The unreacted adiponitrile remains in toluene solution from which it is recovered to recycle back through the reaction. For each kilogram of 5-cyanovaleramide product isolated, approximately 1.25 kg of MnO2 required disposal; this is definitely not a green chemical process!
As an alternative to the chemical synthesis described above, a biochemical synthesis was developed using organisms that had nitrile hydratase enzymes to convert the C≡N functional group to the amide group.2 The microorganism chosen for this conversion was designated Pseudomonaschloroaphis B23. The cells of this organism were immobilized in beads of calcium alginate, the salt of alginic acid isolated from the cell walls of kelp. It was necessary to run the process at 5 ̊C above which temperature the enzyme lost its activity. With this restriction, multiple runs were performed to convert adiponitrile to 5-cyanovaleramide. During these runs, 97% of the adiponitrile was reacted with only 4% of the reaction going to produce byproduct adipamide. The water-based reaction mixture was simply separated mechanically from the calcium alginate beads containing the microorganisms, which were then recycled for the next batch of reactant. The water was distilled off of the product to leave an oil from which the 5-cyanovaleramide product was dissolved in methanol, leaving adipamide and other byproducts behind. In contrast to the enormous amount of waste catalyst produced in the chemical synthesis of 5-cyanovaleramide, only 0.006 kg of catalyst waste residue was produced per kg of product. The waste microbial catalyst was 93% water, so its disposal was not a problem.

