5: Aseptic Technique and Streak Plates
- Page ID
- 110765
By the end of this lab period, you will be able to
- You will be able to define “mixed culture” and “pure culture”.
- You will be able to articulate the principles of aseptic transfer.
- You will know how to aseptically transfer bacteria from one pure culture to another using broths, slants, and deeps.
- You will know the proper way to prepare streak plates to isolate pure cultures from a mixed culture.
Introduction
As we saw in Lab #4, when we take environmental or body part samples, and put them into growth media, more than one type of organism will usually grow. If you put them in liquid media, they will grow together and you’ll have a mixed culture.
When a single organism (species) is present in growth medium, we use the term “pure culture”. Because there are microbes everywhere, special care must be taken to maintain a pure culture and prevent it from becoming contaminated with other organisms. Since bacteria are living organisms, over time they will use up the nutrients that are in culture media. Many bacteria also produce acid as a metabolic byproduct. To maintain a pure culture for longer periods, organisms must be “sub-cultured” by moving them to fresh media.
Types of Media
Microbiologists use a variety of different media to grow microorganisms, and we will be using a wide variety of these media over the course of the semester, including some media that selects for or differentiates between the growth of specific types of bacteria.
However, there are some kinds of non-selective media that allow you to grow a wide variety of microbes. In our microbiology laboratory, we grow most of our organisms in Nutrient Agar (NA/NB) or Trypticase Soy Agar (TSA). Nutrient agar (NA) and Nutrient Broth (NB) use the same formulation, except that agar has been added to form a solid medium to make NA. NA/NB is a simple medium and does not contain a lot of extra nutrients. Because of this, only organisms that are easy to grow will typically thrive. Fortunately, many of the organisms that we use grow well in NA/NB. Trypticase Soy Agar (TSA) contains somewhat more nutrients than NA, and can be used to grow organisms that are more fastidious - that is, require a richer medium with additional nutrients to grow well.
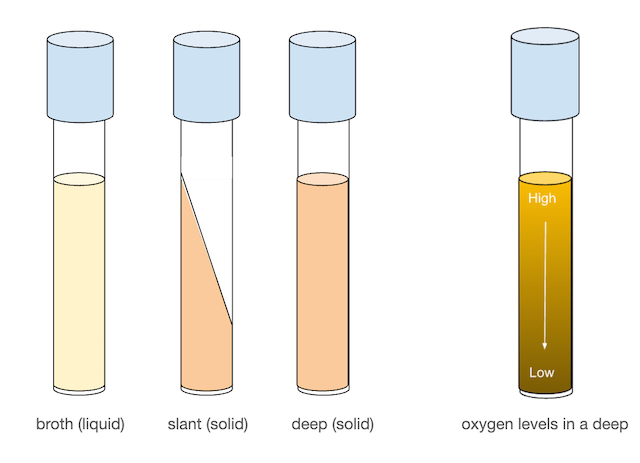
When liquid or solid media is made, it can be prepared in a variety of formats. When a large culture volume is needed, bacteria can be grown in large flasks. However, when only small amounts of bacteria are needed, we typically use culture tubes. Culture tubes can be used to prepare both liquid (broth) media and solid media. Solid media is frequently prepared in flat plates known as Petri plates or Petri dishes when a larger surface is needed. In today’s lab, we will be using Nutrient Broths (NB), Nutrient Agar (NA) Deeps, NA Slants, and NA plates. We will also be using a Trypticase Soy Agar (TSA) plate.
Agar slants, in particular, are very useful for growing and maintaining stock cultures for several weeks. Agar deeps can be used to look at bacterial motility as well as the oxygen dependence of organisms as there is always more oxygen at the surface of the deep than at the middle and bottom. Petri plates are used for the isolation of bacteria, as well as for differentiation and quantification (counting) of bacteria.
Growth in liquid culture
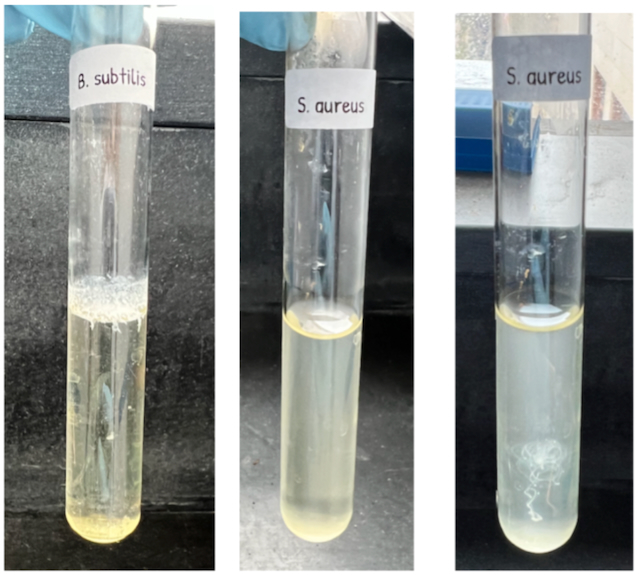
Individual bacteria are far too tiny to see without a microscope! However, in the lab when grown under ideal conditions, we can see bacteria when there are a lot of them. In a liquid culture, bacteria can be seen by the cloudy appearance of the broth after inoculation. Sometimes the entire broth is quite cloudy or turbid. In other situations, bacteria can form a pellicle, which is a floating biofilm of bacteria at the liquid-air interface of the culture. You may also find that bacteria grown in broth need a vigorous shake to redistribute the organisms throughout the tube as most have fallen to the bottom of the tube. This is referred to as “sediment”.
Aseptic Transfers
Today, you will be given some cultures prepared by your instructor and asked to aseptically transfer them to new media - a process called “subculturing”. Your instructor will demonstrate the process for you in class. It is critical that the cultures are transferred into media that has been sterilized and that you use proper aseptic technique in order to avoid contamination.
The steps for making an aseptic transfer are as follows:
Part I
- Label the media that you are inoculating with the date, organism name, and your name.
- Light your Bunsen burner or ethanol lamp to produce a steady flame.
- Sterilize your inoculating loop. Hold it in your dominant hand as you would a pencil (Figure \(\PageIndex{4}\)).
- Grasp the culture tube containing the bacterial culture in your non-dominant hand.
- Using your dominant hand, use your ring finger and pinky to remove the metal culture tube cap (Figure \(\PageIndex{5}\)).
- Pass the top of the glass culture tube through the flame 2 or 3 times. This warms up the air inside the tube, forcing it to expand and move upward, and reduces the likelihood of an environmental organism contaminating the culture (Figure \(\PageIndex{6}\)).
- Using your sterile inoculating loop, collect bacteria from the broth or deep. You need only touch the loop to the bacteria - plenty of organisms will remain on the loop even if you cannot see them (Figure \(\PageIndex{7}\)).
- Pass the top of the glass culture tube through the flame 2 or 3 times again.
- Replace the culture tube cap (it should still be in your dominant hand).
Part II
- Grasp the culture tube containing the fresh media in your non-dominant hand.
- Using your dominant hand, use your ring finger and pinky to remove the metal culture tube cap.
- Pass the top of the glass culture tube through the flame 2 or 3 times.
- Inoculate the new media by touching it with the loop. If this is a slant, place the loop at the bottom of the slant and drag it up over the slant surface.
- Pass the top of the glass culture tube through the flame 2 or 3 times again.
- Replace the culture tube cap (it should still be in your dominant hand).
- Sterilize the inoculating loop.
Preparation of a Streak Plate
What happens when you have two or more organisms mixed together and you want to isolate one or more to grow in a pure culture? In this case, we use a “streak plate” to grow colonies originating from individual bacteria.
Streaking for isolation can be done in several ways, but we will focus on one of the most common methods, the three-sector streak method. When creating a streak plate, the goal is to dilute the bacteria in each step so that each “streak” contains fewer individual organisms than the prior one. Eventually, there are so few bacteria that as you streak, individual organisms are spread out far apart from one another. As they grow in the incubator, these individual organisms will grow into discrete colones originating from a single organism (species).
Steps to make a streak plate:
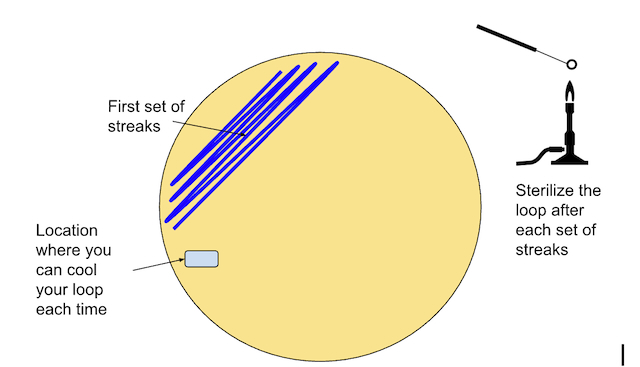
- Sterilize the inoculating loop.
- Touch the loop to the agar surface to cool it down. You may hear a “sizzle” as the hot loop is rapidly cooled by contact with the agar.
- Inoculate the loop by dipping it into broth, or touching it to the surface of a bacterial colony. You don’t need to be able to see any globs of bacteria on the loop! There are plenty there by simply touching the surface once.
- Create the first streaks following the pattern below (Figure \(\PageIndex{9}\))
- Sterilize the inoculating loop again.
- Touch the loop to the agar surface (again) to cool it down.
- Create the second streaks following the pattern below. Be sure to pass the loop through the original (1st) streaks 1-5 times in order to spread the bacteria out further (Figure \(\PageIndex{10}\))
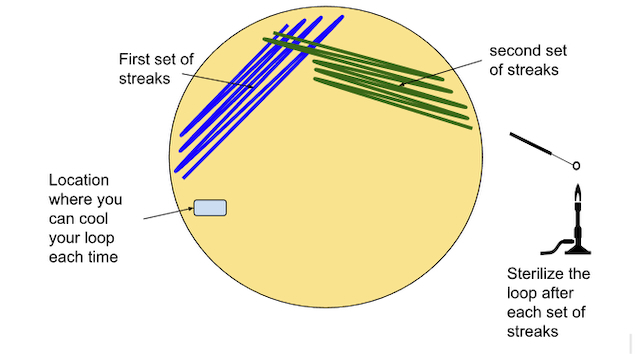
- Sterilize the inoculating loop again.
- Touch the loop to the agar surface (again) to cool it down.
- Create the third streaks following the pattern below. Be sure to pass the loop through the original (2nd) streaks 1-5 times in order to spread the bacteria out further. (Figure \(\PageIndex{11}\))
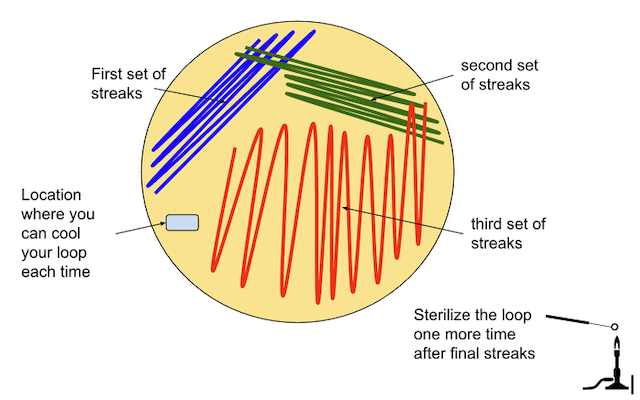
Materials
Per student:
- Two uninoculated Nutrient agar “deeps”
- Two uninoculated Nutrient agar “slants”
- Two uninoculated Nutrient “broths”
- Two Nutrient agar plates
- One TSA plate
- An inoculating loop
- An inoculating needle
Per student pair:
Cultures of:
- Serratia marcescens
- Chromobacterium violaceum
- Bacillus subtilis
- Staphylococcus aureus
- Proteus mirabilis
Experiment
- Label each uninoculated tube/plate with your first and last name, date, and lab section.
- Inoculate two nutrient agar (NA) deeps, two NA slants, and two nutrient broths (NB).
- Subculture S. aureus to a slant and a deep.
- Subculture S. marcescens to a broth and a deep.
- Subculture B. subtilis to a slant and a broth.
- Label each new culture with the name of the organism that you used!
- Prepare two streak plates, using C. violaceum for one, and S. marcescens for the other.
- Prepare a line inoculation on a TSA plate of the organism P. mirabilis.
- Place your new tubes in racks, and loosely place a rubber band around each set of tubes so that they are less likely to get separated. Incubate at 35-37 degrees until the next lab period.
- Incubate your three plates upside down (lids on the bottom) at 35-37 degrees until the next lab period.
- Discard the old cultures. Be sure to clean the labels off of the tubes before placing them upright in the metal bins. Ask for help if you don’t remember how to properly discard your tubes.
Data
No data will be collected this lab period, but we will be using these subcultures next lab period to prepare gram stains. You might find it helpful to consider a few questions.
Questions
- Why do we incubate the new plates upside down (lid on the bottom)?
- Why do we flame the lip of each culture tube before and after inserting an inoculating loop or needle?
- For which type(s) of media do we use an inoculation loop vs an inoculation needle?
- When are the appropriate times to sterilize your loop or needle?
- What is the purpose of a streak plate?
- Why do we cool off the inoculation loop before using it to create streaks on a streak plate?
- Why do we flame the inoculation loop between each set of streaks on a streak plate?
- When you prepare a streak plate, which set of streaks (1, 2, or 3) has the highest bacteria count? Which has the lowest? Why is this important in preparing a streak plate?

