4.2: Nutrients
- Last updated
- Save as PDF
- Page ID
- 22453
Fighting Phytochemicals
Many wars have been fought to acquire these spices from India. Chemicals and oils in the spices infuse specific smells and tastes in Indian cuisine. Food and culture are intertwined, and people bring their culture with them when they settle in a foreign country. Sometimes their culture is accepted, and sometimes it becomes a cause of discrimination that people have to face for embracing their culture.
This colorful display of Indian spices is not just pretty to look at, the items pictured are also rich in phytochemicals. Phytochemicals are a large group of recently discovered chemicals, such as oils and colors, that occur naturally in plants. Many of them are known to protect plants by fighting off insect attacks and infectious diseases. Phytochemicals in the food we eat may also be needed to help keep us healthy. If so, some nutritionists think they should be classified as nutrients.

What Are Nutrients?
Nutrients are substances the body needs for energy, building materials, and control of body processes. There are six major classes of nutrients based on biochemical properties: carbohydrates, proteins, lipids, water, vitamins, and minerals. Fiber, which consists largely of nondigestible carbohydrates, is sometimes added as the seventh class of nutrients.
Besides the biochemical classification of nutrients, nutrients are also categorized as either essential or nonessential nutrients. Essential nutrients cannot be synthesized by the human body, at least not in sufficient amounts for normal functioning, so these nutrients must be obtained from food. Nonessential nutrients, in contrast, can be synthesized in the body in sufficient quantities for normal functioning, although they are generally obtained from food as well. Except for dietary fiber, all dietary carbohydrates are considered nonessential. Every other major class of nutrients contains multiple essential compounds. For example, there are nine essential amino acids, at least two essential fatty acids, and many essential vitamins and minerals. Water and fiber are also essential nutrients.
The major classes of nutrients are also categorized as macronutrients or micronutrients depending on how much of them the body needs.
Macronutrients
Macronutrients are nutrients that the body needs in relatively large amounts. They include carbohydrates, proteins, lipids, and water. All macronutrients except water are used by the body for energy, although this is not their sole physiological function. The energy provided by macronutrients in food is measured in kilocalories, commonly called Calories, where 1 Calorie is the amount of energy needed to raise 1 kilogram of water by 1 degree Celsius.
Carbohydrates
Carbohydrates are organic compounds made up of simple sugars (as in the cotton candy pictured in Figure \(\PageIndex{2}\)). Carbohydrates are classified by the number of sugars they contain as monosaccharides (one sugar), such as glucose and fructose; disaccharides (two sugars), such as sucrose and lactose; and polysaccharides (three or more sugars), including starch, glycogen, and cellulose (the main component of dietary fiber). Dietary carbohydrates come mainly from grains, fruits, and vegetables. All digestible carbohydrates in the diet are used by the body for energy. One gram of dietary carbohydrates provides 4 Calories of energy. Fiber, such as the cellulose in plant foods, cannot be digested by the human digestive system, so most of it just passes through the digestive tract. Although it does not provide energy as other carbohydrates do, it is nonetheless considered an essential nutrient for its physiological roles. There are two types of fiber in many plant foods: soluble fiber and insoluble fiber.
.jpg?revision=1&size=bestfit&width=231&height=347)
Soluble fiber consists of nondigestible complex plant carbohydrates that dissolve in water, forming a gel. This type of dietary fiber thickens and slows the movement of chyme through the small intestine and thereby slows the absorption of glucose into the blood. The consistency of food after it has been mechanically digested in the stomach is referred to as chyme. This may lessen insulin spikes and the risk of type 2 diabetes. Soluble fiber can also help lower blood cholesterol. Good dietary sources of soluble fiber include oats, apples, and beans.
Insoluble fiber consists mainly of cellulose and does not dissolve in water. As insoluble fiber moves through the large intestine, it stimulates peristalsis. Peristalsis is the involuntary constriction of the smooth muscle of the GI tract that pushes the food content in the tract. This keeps food wastes moving and helps prevent constipation. The insoluble fiber in the diet may also lessen the risk of colon cancer. Good dietary sources of insoluble fiber include cabbage, bell peppers, and grapes.
Proteins
Proteins are organic compounds made up of amino acids. You may think of meat and fish as major sources of dietary proteins — and they are — but there are many good plant sources as well, including soybeans (see the figure below) and other legumes. Proteins in food are broken down during digestion to provide the amino acids needed for protein synthesis. Proteins in the human body are the basis of many body structures, including muscles and skin. Proteins also function as enzymes that catalyze biochemical reactions, hormones that regulate body functions in other ways, and antibodies that help fight pathogens. Any amino acids from food that are not needed for these purposes are excreted in the urine, converted to glucose for energy, or stored as fat. One gram of protein provides 4 Calories of energy.
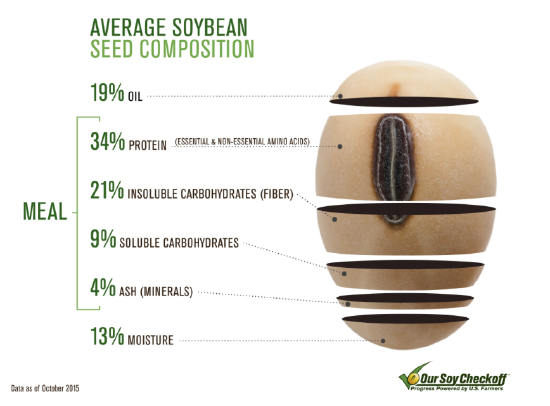
The most important aspect of protein structure from a nutritional standpoint is amino acid composition. About 20 amino acids are commonly found in the human body, of which about 11 are nonessential because they can be synthesized internally. The other 9 amino acids are essential amino acids that must be obtained from dietary sources. Essential amino acids are phenylalanine, valine, threonine, tryptophan, methionine, leucine, isoleucine, lysine, and histidine. Animal proteins such as meat and fish are concentrated sources of all 9 essential amino acids, whereas plant proteins may have only trace amounts of one or more essential amino acids.
Lipids
Lipids, commonly called fats, are organic compounds made up mainly of fatty acids. Fats in foods (Figure \(\PageIndex{4}\)), as well as fats in the human body, are typically triglycerides (three fatty acids attached to a molecule of glycerol). Fats provide the body with energy and serve other vital functions, including helping to make and maintain cell membranes and functioning as hormones. When used for energy, one gram of fat provides 9 Calories of energy.

Saturated vs Unsaturated Fats
Fats are classified as either saturated or unsaturated depending on the type of bonds in their fatty acids.
- In saturated fats, carbon atoms share only single bonds, so each carbon atom is bonded to as many hydrogen atoms as possible. Saturated fats tend to be solids at room temperature. Most saturated fat in the diet comes from animal foods, such as meat and butter.
- In unsaturated fats, at least one pair of carbon atoms share a double bond, so these carbon atoms are not bonded to as many hydrogen atoms as possible. Unsaturated fats with just one double bond are called monounsaturated fats. Those with multiple double bonds are called polyunsaturated fats. Unsaturated fats tend to be liquids at room temperature. Unsaturated fats in the diet come mainly from certain fish such as salmon and from plant foods such as seeds and nuts.
Essential Fatty Acids
Most fatty acids are not essential. The body can make them as needed, generally from other fatty acids, although this takes energy. Only two fatty acids are known to be essential, called omega-3 and omega-6 fatty acids. They cannot be synthesized in the body, so they must be obtained from food. The most commonly used cooking oils in processed foods are rich in omega-6 fatty acids, so most people get plenty of these fatty acids in their diet. Omega-3 fatty acids are not as prevalent in foods, and most people do not get enough of them in food. Good food sources of omega-3 fatty acids include oily fish such as salmon, walnuts, and flax seeds.
Trans Fats
Trans fats are unsaturated fats that contain types of bonds that are rare in nature. Trans fats are typically created in an industrial process called partial hydrogenation. They may be used in a variety of processed foods (such as those shown in Figure \(\PageIndex{5}\)) because they tend to have a longer shelf life without going rancid. Trans fats are known to be detrimental to human health.

Water
Water is essential to life because biochemical reactions take place in water. Water is continuously lost from the body in multiple ways, including in urine and feces, during sweating, and as water vapor in exhaled breath. This constant loss of water makes water an essential nutrient that must be replenished often.
Too little water is called dehydration. It can cause weakness, dizziness, and heart palpitations. Severe dehydration can lead to death. It is easy to become dehydrated in hot weather, especially when exercising. It is more difficult to consume too much water, but overhydration is also possible. It can result in water intoxication, a serious and potentially fatal condition.
Micronutrients
Micronutrients are nutrients the body needs in relatively small amounts. Micronutrients do not provide energy. Instead, they are necessary for the biochemical reactions of metabolism, among other vital functions. They include vitamins, minerals, and possibly phytochemicals as well.
Vitamins
Vitamins are organic compounds that generally function as coenzymes. A coenzyme is a “helper” molecule that is required for a protein enzyme to work. In this capacity, vitamins play many roles in good health, ranging from maintaining normal vision (vitamin A) to help the blood clot (vitamin K). Some functions of these and several other vitamins are listed in the table below. Most vitamins are essential nutrients and must be obtained from food. Fruits, vegetables, meat, and fish are all high in one or more essential vitamins. There are only a few nonessential vitamins. Vitamins B7 and K are produced by bacteria in the large intestine, and vitamin D is synthesized in the skin when it is exposed to UV light
| Vitamin | Function |
|---|---|
| A | normal vision |
| B1 (thiamin) | production of cellular energy from food |
| B3(niacin) | cardiovascular health |
| B7 (biotin) | support of carbohydrate, protein, and fat metabolism |
| B9 (folic acid) | fetal health and development |
| B12 | normal nerve function and production of red blood cells |
| C | making connective tissue |
| D | healthy bones and teeth |
| E | normal cell membranes |
| K | blood clotting |
Minerals
Minerals are inorganic chemical elements that are necessary for normal body processes and good health. Because they are inorganic and not synthesized biologically, all nutrient minerals are considered essential nutrients.
Several minerals are needed in relatively large quantities (> 150 mg/day), so they are sometimes referred to as macrominerals or bulk minerals. They include:
- calcium, which is needed for bone strength, neutralizing acidity in the digestive tract, and nerve and cell membrane functions. Dairy products are good sources of calcium.
- magnesium, which is needed for strong bones, maintaining pH, processing ATP, and other functions. Green leafy vegetables, bran, and almonds are high in magnesium.
- phosphorus, which is needed for bone strength, energy processing, pH regulation, and phospholipids in cell membranes. Milk and meat are good sources of phosphorus.
- sodium, which is needed to regulate blood volume, blood pressure, water balance, and pH. Most processed foods have added sodium. A salt shaker is another common source of sodium.
- chloride, which is needed for the production of hydrochloric acid in the stomach and for cell membrane transport. Chloride in table salt added to processed foods provides plenty of chloride in most diets.
- potassium, which is needed for the proper functioning of the heart and nerves, water balance, and pH. Many fruits and vegetables are high in potassium.
- sulfur, which is needed for the synthesis of many proteins. Meat and fish are good sources of sulfur.
Other minerals are needed in much smaller quantities (≤150 mg/day), so they are often referred to as trace minerals. The table below lists several trace minerals and some of their functions. Good dietary sources of trace minerals include whole grains, seafood, fruits, vegetables, nuts, and legumes.
| Trace Mineral | Function |
|---|---|
| Cobalt | synthesis of vitamin B12 by gut bacteria |
| Copper | component of many enzymes |
| Chromium | metabolism of sugar |
| Iodine | synthesis of thyroid hormones |
| Iron | component of hemoglobin and many enzymes |
| Manganese | processing of oxygen |
| Molybdenum | component of several enzymes |
| Selenium | component of oxidases (antioxidants) |
| Zinc | component of several enzymes |
Phytochemicals
The naturally occurring, disease- and pest-fighting plant chemicals known as phytochemicals are commonly consumed in plant foods, particularly spices and fresh vegetables and fruits. Besides fighting attacks on plants, many phytochemicals give plants their distinctive colors and characteristic flavors and aromas. Phytochemicals are the reason that blueberries are blue (Figure \(\PageIndex{6}\)) and that garlic has its characteristically strong, pungent taste and smell. There are known to be as many as 4,000 different phytochemicals in plants. Preliminary evidence suggests that certain phytochemicals in the diet help protect human health. For example, some phytochemicals may act as antioxidants that counter cancer-causing free radicals. Research on phytochemicals is still relatively young, so time will tell whether they will eventually be classified as micronutrients.

Review
- What are the nutrients?
- List the six major classes of nutrients based on biochemical properties.
- Compare and contrast essential and nonessential nutrients.
- Identify macronutrients.
- Which nutrients are classified as micronutrients? Why?
- Describe carbohydrates, state how much energy they provide, and list good food sources of carbohydrates.
- If fiber in food cannot be digested, why is it considered a nutrient?
- Describe proteins, state their general uses in the human body, and identify food sources that are high in proteins. How much energy do proteins provide?
- Describe lipids, identify how much energy they provide, and state their general uses in the human body.
- Distinguish between saturated, unsaturated, and trans fats.
- Water provides no energy or materials the body needs for building or controlling body processes. Why is it considered a nutrient?
- What are vitamins? What is the general role of most vitamins? Which vitamins are not essential nutrients? Why?
- What are the dietary minerals? Give examples of macrominerals and trace minerals.
- What are phytochemicals? What are good food sources of phytochemicals?
- Which of the following are inorganic substances?
- Vitamins
- Minerals
- All micronutrients
- A and B
Explore More
Dietary intake of the bioactive components within fruits and vegetables has been shown to have chemopreventative effects. Many flavonoids have been found to be cytotoxic to cancer cells. Learn more here:
Attributions
- Indian Spices by Joe mon bkk, CC BY-SA 4.0 via Wikimedia Commons
- Cotton candy fan by college.library, licensed CC BY 2.0 via Wikimedia Commons
- Soybean Composition Infographic by United Soybean Board, licensed CC BY 2.0 via Wikimedia Commons
- Butter and oil by National Cancer Institute, public domain via Wikimedia Commons
- Avoiding trans fat by The U.S. Food and Drug Administration, public domain via Wikimedia Commons
- Weather tomorrow - sunny with plentiful blueberries by Gordana Adamovic-Mladenovic, licensed CC BY 2.0 via Wikimedia Commons
- Text adapted from Human Biology by CK-12 licensed CC BY-NC 3.0


