8.5: Redox zonation
- Page ID
- 131871
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Patterns in the redox chemistry of many environments indicate that the electron acceptors used by chemotrophic respiration tend to be depleted sequentially in order of decreasing energy yield (Fig. \(8.3\)). Oxygen is typically more thermodynamically favorable as an electron acceptor than nitrate. Therefore, microbial communities tend to consume oxygen before nitrate. Where oxygen is depleted, the community may primarily respire nitrate if it is available, and where nitrate is depleted, respiring microorganisms may move on to less favorable electron acceptors such as ferric iron minerals, sulfate, and carbon dioxide. Patterns in chemistry consistent with this redox zonation have been observed in aquifers, soils, marine sediments, microbial biofilms, and more (Champ et al., 1979; Froelich et al., 1979; Patrick Jr. and Henderson, 1981; Stewart and Franklin, 2008).
Zonation in electron acceptor use has been interpreted to reflect competition between microbes for electron donors (Chapelle and Lovley, 1992; Hoehler et al., 1998; Lovley and Phillips, 1987). Microorganisms that can capture more energy from their reactions can have competitive advantages over those that capture less, including more energy for growth and maintenance and faster reactions (Jin, 2012; Jin and Bethke, 2007; Lovley and Goodwin, 1988; Roden and Jin, 2011). As such, the microbial community uses more energetically favorable reactions preferentially to less favorable reactions. In other words, the community works its way along the thermodynamic ladder from most to least favorable reactions.
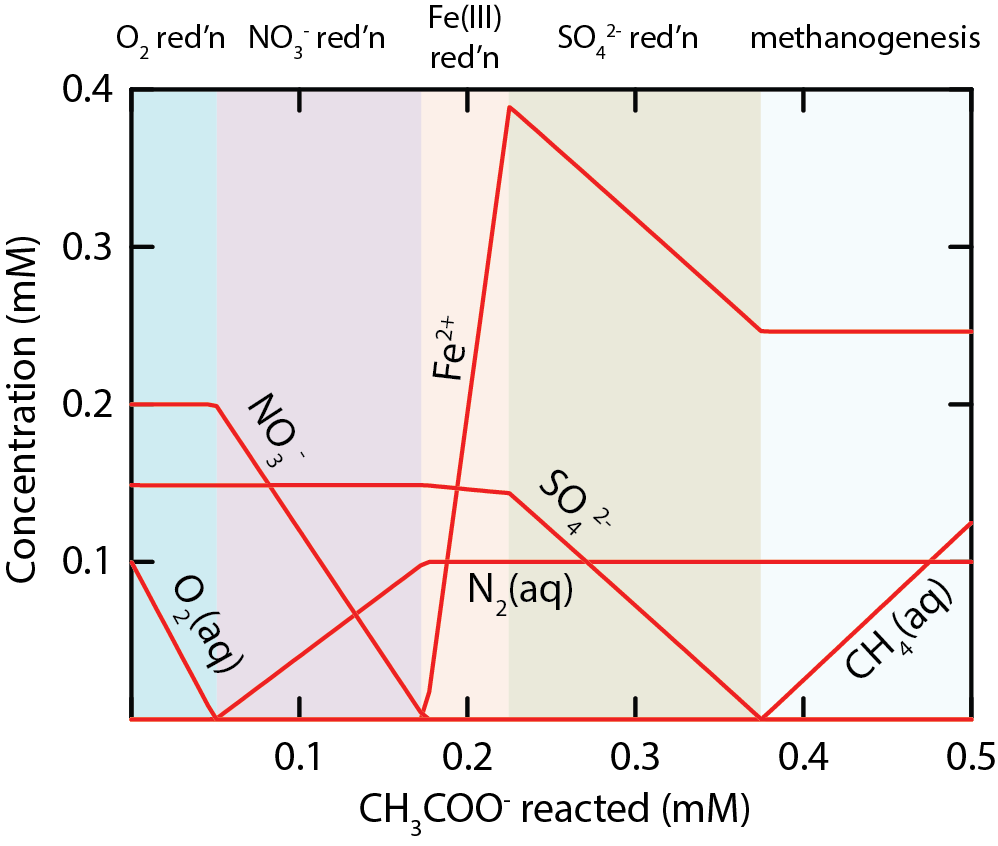
https://commons.wikimedia.org/wiki/File:Redox_zonation.png
This model can allow us to define an overall framework for predicting microbial processes based on redox chemistry, which we do later in this chapter. However, it is also recognized that this redox zonation model is an oversimplification (Bethke et al., 2011; Konhauser, 2007), and it is important to understand some of the reasons why that is the case if we are to use such a framework effectively.
First, free energy yields of microbial reactions vary with environmental conditions. As a result, a ranking of reactions in terms of energy yield can differ from one environment to the next. Moreover, energy yields of different reactions can overlap, obscuring any thermodynamic hierarchy that might exist otherwise. As an example, pH affects the amount of energy released by reduction of ferric iron in (oxyhydr)oxide minerals, as shown in Figure \(8.2\text{A}\). As pH increases, the reaction becomes less favorable. Because of this relationship, iron reduction can be more favorable than sulfate reduction and methanogenesis if the pH of the environment is acidic. However, where pH is neutral or basic, sulfate reduction and methanogenesis can release as much or more energy than iron reduction (Bethke et al., 2011; Jin and Kirk, 2018; Kirk et al., 2013; Marquart et al., 2019; Paper et al., 2021; Postma and Jakobsen, 1996).
Secondly, microbial environments are typically heterogenous in chemical and physical properties. The properties of microbial environments can change significantly on scales that are small relative to human perception but enormous to a microbe. We recall from Chapter 3 that microorganisms are very small. If you lined up 10,000 cells each with a length of 1 µm, the line of cells would only extend for 1 cm. However, properties of many microbial environments can change significantly within a centimeter. Therefore, the likelihood that more than one form of microbial respiration is present in a microbial habitat increases quickly with the scale of observation.
As an example, we can consider the existence of anoxic microenvironments with upland soils. In terms of bulk chemical properties, upland soils are often considered to be well aerated and dominated by aerobic microbial respiration. However, anoxic microenvironments can exist within upland soils and play an important role in organic carbon storage (Keiluweit et al., 2017, 2016). In upland soils, macropores \((> 50 \ \mu\text{m})\) surround aggregates and soil peds. Gas and water flow can advectively transport oxygen through the macroporesm, but transport into the interior of an aggregate or ped may be limited to diffusion. If demand for electron acceptors exceeds oxygen supply in the aggregate or ped interior, then anoxic conditions can develop there, and anaerobic microorganisms may be active. Thus, structural and compositional heterogeneity within upland soils can create complex patterns of redox chemistry.
A third source of complexity stems from the fact that the redox state of an environment is not necessarily constant over time. As such, the spatial distribution of redox zones can vary. As an example, variation in water saturation in soil can decrease oxygen supply relative to electron acceptor demand, causing development of anoxic conditions, as discussed in Chapter 6. Therefore, where water saturation varies over time, the redox state of the soil can also be temporally variable (Fig. \(6.3\text{B}\)).
Fourth, it is critical to recognize that thermodynamics is not the only thing that controls the distribution of microbial reactions. Properties of the environment such as temperature, salinity, and the form and abundance of electron donors influence microbial populations. Competition between different groups of microorganisms for electron donors has the potential to be influenced not only by thermodynamics but also kinetics (Bethke et al., 2008). Moreover, interactions between microbial groups extend beyond competition, as discussed in Chapter 10. Some groups coexist and can even cooperate with one another, for example, which influences their ability to grow within an environment. Such complexity is not well captured by a framework primarily based on the thermodynamic ladder.
Calculate the free energy change \(\left(\Delta G_{r}\right)\) for the following reactions. Assume environmental conditions consistent with those listed in Table \(8.1\) and an activity of \(1\) for water. Obtain \(\log K\) values for each reaction in Appendix A. Which of these reactions has the potential to give microorganisms the biggest advantage in terms of energy?
\[\begin{align*} & \text{CH}_{3} \text{COO}^{-} + 2 \ \text{O}_{2} \longleftrightarrow 2 \ \text{HCO}_{3}^{-} + \text{H}^{+} \\ & \text{CH}_{3} \text{COO}^{-} + 0.6 \ \text{H}^{+} + 1.6 \ \text{NO}_{3}^{-} \longleftrightarrow 2 \ \text{HCO}_{3}^{-} + 0.8 \ \text{N}_{2} \ (aq) + 0.8 \ \text{H}_{2} \text{O} \\ & \text{CH}_{3} \text{COO}^{-} + \text{H}_{2}\text{O} \longleftrightarrow \text{HCO}_{3}^{-} + \text{CH}_{4} \ (aq) \end{align*}\]
How does the source of ferric iron affect the energy available for microbial iron reduction? To find out, calculate the free energy change \(\left(\Delta G_{r}\right)\) for the following reactions. Assume environmental conditions consistent with those listed in Table \(8.1\) and an activity of \(1\) for water. Obtain \(\log K\) values for each reaction in Appendix A. Which of these reactions has the potential to give microorganisms the biggest advantage in terms of energy?
\[\begin{align*} & \text{CH}_{3} \text{COO}^{-} + 15 \ \text{H}^{+} + 8 \ \text{Fe(OH)}_{3} \longleftrightarrow 2 \ \text{HCO}_{3}^{-} + 20 \ \text{H}_{2} \text{O} + 8 \ \text{Fe}^{2+} \\ & \text{CH}_{3} \text{COO}^{-} + 15 \ \text{H}^{+} + 8 \ \text{Goethite} \longleftrightarrow 2 \ \text{HCO}_{3}^{-} + 0.8 \ \text{N}_{2} \ (aq) + 0.8 \ \text{H}_{2}\text{O} \\ & \text{CH}_{3} \text{COO}^{-} + 15 \text{H}^{+} + 4 \ \text{Hematite} \longleftrightarrow 2 \ \text{HCO}_{3}^{-} + 8 \ \text{H}_{2} \text{O} + 8 \ \text{Fe}^{2+} \end{align*}\]
Repeat the calculation for Practice \(8.5.2\) with all parameters the same except increase the pH from 6 to pH 8. Are all three ferric iron sources still potentially useful for dissimilatory metabolism? What do the results suggest about the pattern of redox zonation in systems with pH 8?


