1.14: Acid-Fast Stain
- Page ID
- 90560
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Describe the difference between acid-fast bacteria and non-acid-fast bacteria.
- Explain how the acid-fast stain works comparing acid-fast and non-acid fast bacteria.
- Identify the genera of bacteria that are acid-fast and two examples of diseases caused by these species.
- Differentiate the cell wall structures of acid-fast and non-acid fast bacteria.
- Successfully execute an acid-fast stain and interpret the results.
Acid Fast Stain
Acid fast stain is a differential stain used to identify acid-fast organisms such as members of the genus Mycobacterium. Acid-fast microorganisms are characterized by wax-like, nearly impermeable cell walls; they contain mycolic acid and large amounts of fatty acids, waxes, and complex lipids. This type of cell wall is resistant to most compounds, therefore acid-fast microorganisms require a special staining technique.
The ability of the bacteria to resist decolorization with acid-alcohol confers acid-fastness to the bacterium. Acid-fast bacteria, of which there are very few---the major genus Mycobacterium, have a high concentration of mycolic acid, a lipid, in their cell walls. Although difficult to stain, once the stain goes into the cell wall, the cell will not de-stain or decolorize easily. The ability of the bacteria to resist decolorization with acid-alcohol confers acid-fastness to the bacterium. The phenol in the carbol fuchsin facilitates the dye going into the waxy wall of the bacterium. Acid-fast bacteria stain poorly with the Gram stain procedure, appearing weakly Gram-positive or Gram-variable. They are usually characterized using the acid-fast staining procedure.
Steam is used to get the carbol fuchsin primary dye to go into the cell wall. Once in, it will not come out: But the acid-alcohol decolorizer will take it out of the non-acid fast cell walls since the primary dye does not bind strongly to the cell wall. Non-acid fast bacteria will also take up the carbol fuchsin, but the acid alcohol decolorizer will remove it from wall since the primary dye does not bind strongly to the cell wall.
The primary stain used in acid fast staining, carbol fuchsin, is lipid-soluble and contains phenol, which helps the stain penetrate the cell wall. This is further assisted by the addition of heat in the form of heat (steam). Steam helps to loosen up the waxy layer and promotes entry of the primary stain inside the cell. The smear is then rinsed with a very strong decolorizer (acid-alcohol), which strips the stain from all non-acid-fast cells but does not permeate the cell wall of acid-fast organisms. The decolorized non-acid-fast cells then take up the counterstain, which in our case is methylene blue.
Acid-fastness is an uncommon characteristic shared by the genera Mycobacterium and Nocardia (weakly acid-fast). Because of this feature, this stain is extremely helpful in identification in diseases caused by acid-fast bacteria, particularly tuberculosis and leprosy. In addition, the stain is used to determine the presence of acid-fast bacteria from lung tissue in patients undergoing antibiotic therapy.
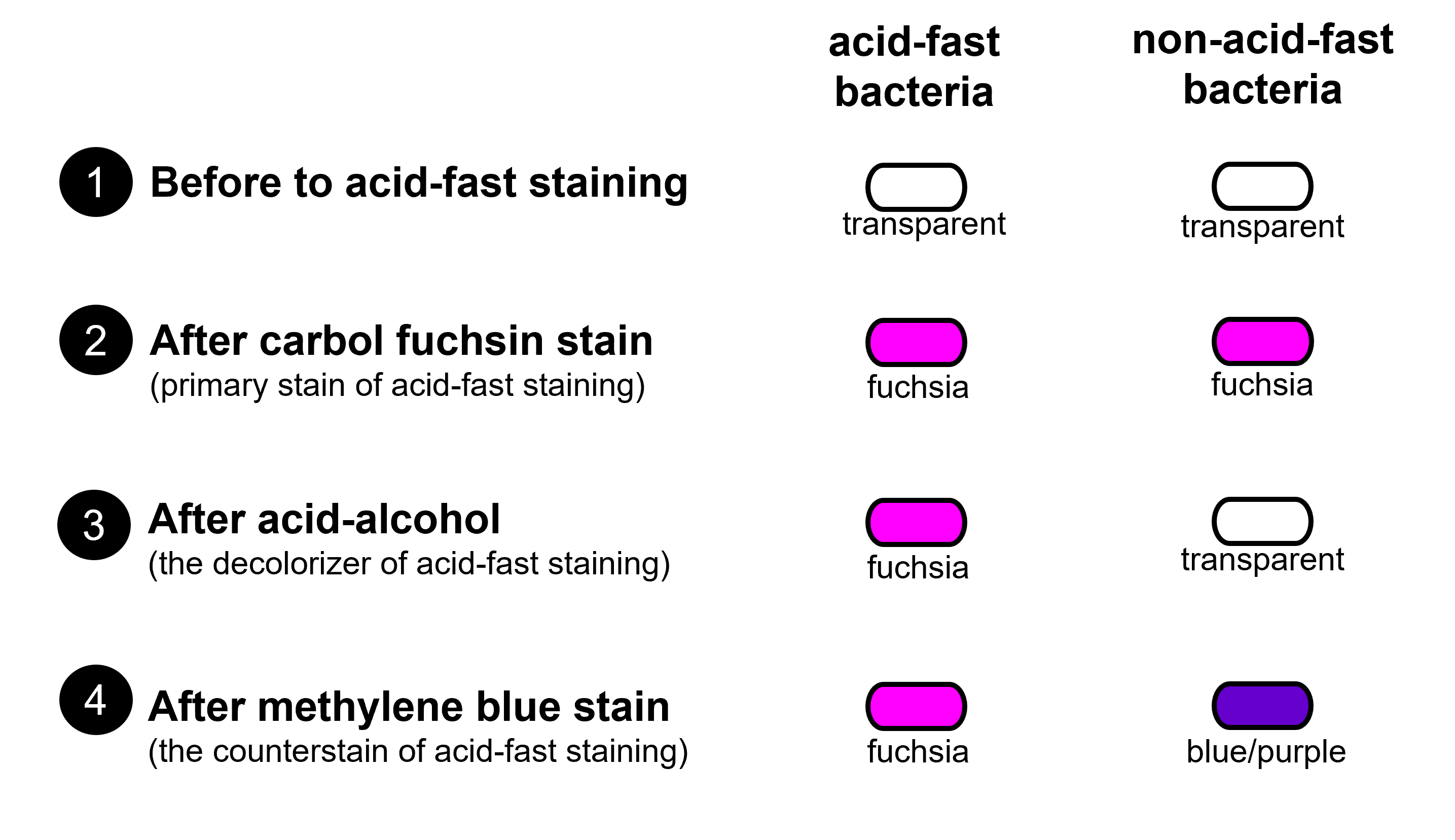
Figure 1: Diagram shows how cells change color during the steps of the acid-fast stain by comparing acid-fast bacteria and non-acid-fast bacteria. Acid-fast bacteria and non-acid-fast bacteria begin transparent. After staining with carbol fuchsin, acid-fast and non-acid-fast bacteria both appear the color fuchsia (a pinky-purple color). After the decolorization step, acid-fast bacteria retains the carbol fuchsin and remains fuchsia and the non-acid fast bacteria looses the color from the carbol fuchsin becoming mainly transparent. Methylene blue counterstain causes non-acid-fast bacteria to stain blue or bluish-purple, whereas acid-fast bacteria remain fuchsia.
Structure and Composition of the Acid-Fast Cell Wall
The mycobacterial cell wall resembles both the Gram-positive and Gram-negative cell walls by having a peptidoglycan layer nearly as thick as Gram-positive cell walls and an outer, waxy layer mimicking the outer membrane of Gram negative cell walls. The cell wall of mycobacteria plays a key role in intrinsic antibiotic resistance and virulence (Forrellad et al. 2013; Becker and Sander 2016).
Acid-fast bacteria are Gram-positive, but in addition to peptidoglycan, the outer membrane or envelope of the acid-fast cell wall of contains large amounts of glycolipids, especially mycolic acids that make up approximately 60% of the acid fast cell wall in the genus Mycobacterium.
The following is a list of the layers of the acid-fast cell wall beginning with the innermost layer (nearest to the plasma membrane):
- Layer 1: Layer of peptidoglycan
- Layer 2: Layer of arabinogalactan (a long carbohydrate composed of the sugars arabinose and galactose)
- Layer 3: Outer membrane containing mycolic acids
- Layer 4: Outer surface is studded with surface proteins that differ with the strain and species of the bacterium
Like the outer membrane of the Gram negative cell wall, porins are required to transport small hydrophilic molecules through the outer membrane of the acid-fast cell wall.
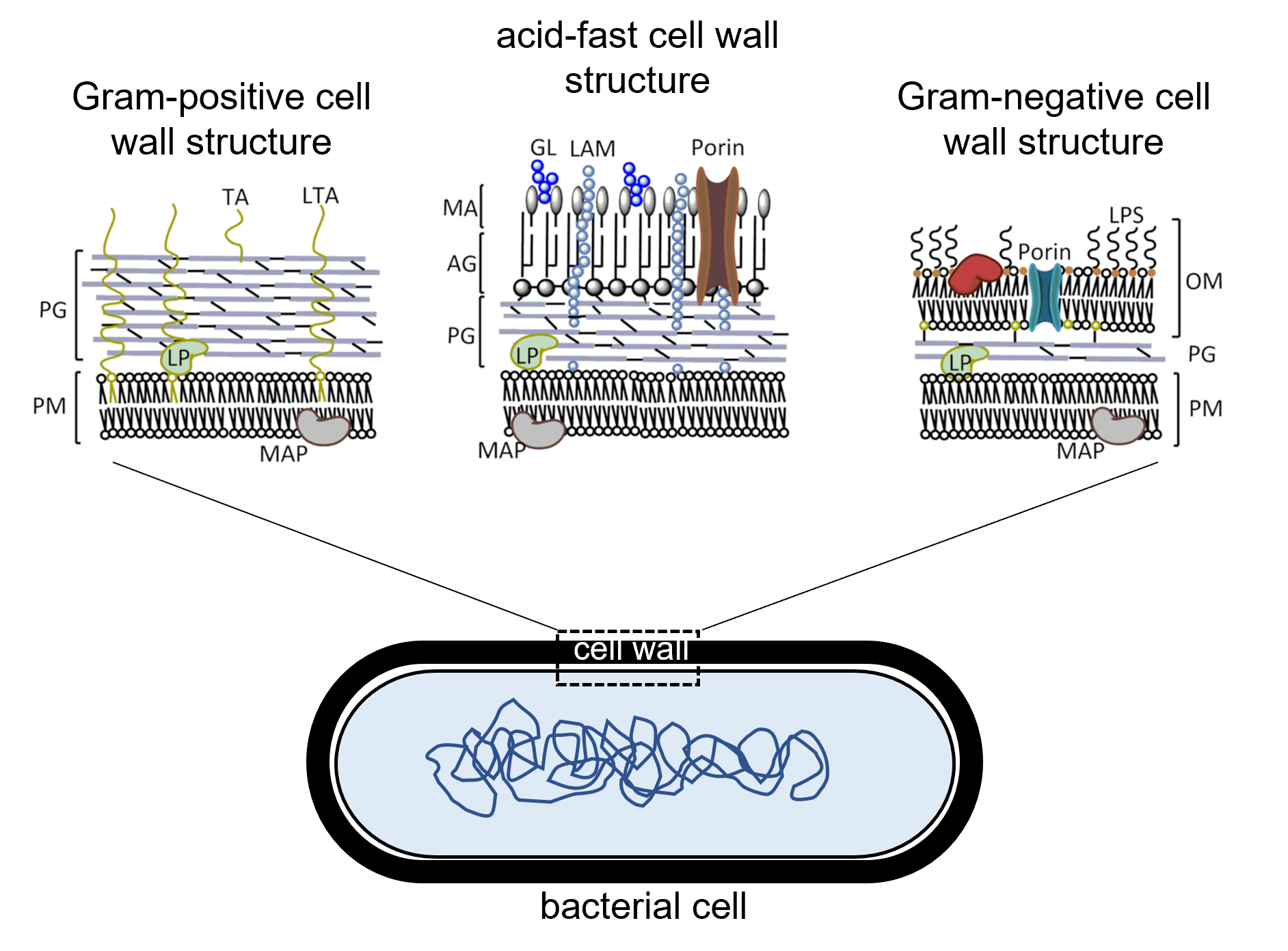
Figure 2: Comparison of the structures of different types of bacterial cell walls. (Left) Gram-positive cell walls consist of a thick layer of peptidoglycan (PG) outside of the cell's plasma membrane (PM) with teichoic acid (TA) and lipoteichoic acid embedded across the peptidoglycan layer. (Right) Gram-negative cell walls consist of a thin layer of peptidoglycan (PG) outside of the cell's plasma membrane (PM). Gram-negative cell walls have an outer membrane (OM) beyond the peptidoglycan layer containing lipopolysaccharide (LPS) as well as porin channels that enable some materials to pass across the outer membrane. (Center) Acid-fast cell walls have a moderately thick layer of peptidoglycan (PG) outside of the plasma membrane. A layer of arabinogalactin (AG) is outside of the peptidoglycan layer and beyond the AG layer is mycolic acid (MA) containing lipoarabinomannan (LAM) and glycolipid (GL). In all three cell wall types, lipoprotein (LP) is also found.
Lab Instructions
- Label a slide and draw a circle on the center of the slide with a wax pencil.
- Prepare an emulsion on the slide with 4 loopfuls of Staphylococcus epidermidis from a broth culture onto the slide (these will be your non-acid-fast bacteria).
- Then, add one loopful of Mycobacterium chelonae (these are your acid-fast bacteria) and mix the two bacteria together.
- Allow the slide(s) to air dry on the slide warmers (while these slides are drying).
- Once the liquid has completely evaporated, heat fix the bacteria by passing it through your flame three times.
- Make sure the slide rack on top of your beaker is completely level. Then, bring your water to boil while the slides are drying. You only need about 200 milliliters of water. If you add more, you will be waiting all lab period for your water to boil.
- Once the water is boiling, place your slide on the slide rack above the boiling water.
- Cover the area of your smear on the slide with a square piece of paper towel. Cut the paper towel to make sure none of the paper is hanging off the slide.
- Carefully apply the carbol fuchsin stain to the paper towel. If a stain appears in the water you are boiling, please stop and discard the stained water in the liquid waste disposal. The fumes from carbol fuchsin can be toxic.
- Steam with the stain on the slide for 7 minutes while continuously applying more stain so the paper square never dries out.
- Gently remove the paper with forceps and discard it in the small waste paper cup that will be provided on your bench. Then, rinse the slide with water.
- Put the slide over the staining basin and gently rinse with water.
- Decolorize with 6 drops of acid-alcohol (not ethanol from the Gram stain kit), then rinse with water.
- Counterstain with methylene blue for 2 minutes.
- Rinse with water and blot dry with bibulous paper (do not use the slide warmer).
- Examine under the 100x objective lens with oil immersion and record your results.
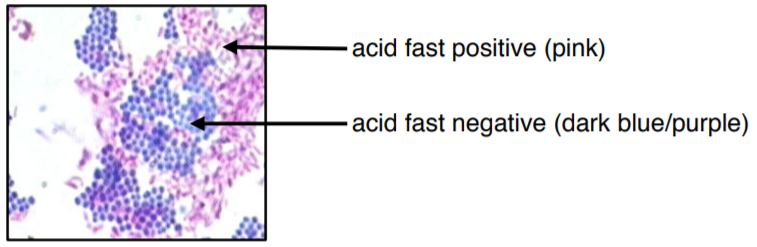
Figure 3: Microscopic image of two different species mixed together with acid fast stain. The acid-fast bacteria appears fuchsia or pinky-purple. The non-acid-fast bacteria appears dark blue or blue-purple. The colors in this image may be slightly off due to printing/copying.
Results & Analysis
- Take a photo of the cells or make an illustration of them.
- Was your acid-fast stain successful? How do you know?
- What color are the acid-fast bacteria?
- What color are non-acid-fast negative bacteria?
- Describe the structure of the cell walls of bacteria that appear fuchsia with the acid-fast stain.
- Describe what you know about the structure of the cell walls of bacteria that appear blue/purple with the acid-fast stain.
- What is the medical importance of using the acid-fast stain (what diseases/infections can it help to identify and how might it relate to virulence and antibiotic resistance)?
Works Cited
- Becker K, Sander P. Mycobacterium tuberculosis lipoproteins in virulence and immunity–fighting with a double‐edged sword. FEBS Lett. 2016;590:3800–19.
- Forrellad MA, Klepp LI, Gioffré A et al. . Virulence factors of the Mycobacterium tuberculosis complex. Virulence. 2013;4:3–66.
Attributions
- Acid Fast Stain by Jackie Reynolds
- Cell wall peptidoglycan in Mycobacterium tuberculosis: An Achilles’ heel for the TB-causing pathogen by Arundhati Maitra et al. is licensed under CC BY 4.0
- General Microbiology Lab Manual (Pakpour & Horgan) by Nazzy Pakpour & Sharon Horgan is licensed under CC BY-SA 4.0
- The Acid Fast Cell Wall by Gary Kaiser is licensed under CC BY 4.0


