1.26: Nitrate Reduction
- Page ID
- 90572
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)- Explain how different bacterial species can conduct nitrate reduction including the possible reactions, possible products, and the name of the enzyme that reduces nitrate.
- Tell the role of nitrate in anaerobic respiration of the species that reduce nitrate.
- Successfully conduct and interpret results of the nitrate reduction test.
- Explain why the nitrate reduction test is useful for characterizing and differentiating species of bacteria.
Nitrate Reduction
Nitrate (NO3) is a nitrogen-containing molecule that can be reduced by some bacterial species, but not others. Therefore, examining the ability of bacterial species to conduct nitrogen reduction is useful for characterizing and identifying bacterial species.
Reduction of nitrate generally occurs during anaerobic respiration in which an organism derives its oxygen from nitrate to serve as the final electron acceptor to remove electrons from the electron transport chain. In some species, nitrate is reduced to nitrite leaving nitrite as evidence of the process:
NO3 (nitrate) → NO2 (nitrite)
In other species, nitrate is first reduced to nitrite and then to ammonia:
NO3 (nitrate) → NO2 (nitrite) → NH3 (ammonia)
In still other bacterial species, denitrification occurs where nitrate is reduced completely to N2 gas and becomes largely unavailable to most living things as a nitrogen source:
NO3 (nitrate) → NO2 (nitrite) → N2 (nitrogen gas)
Nitrate broth is used to determine if an organism can reduce nitrate. Some bacteria can reduce nitrate (NO3) to nitrite (NO2) by producing the enzyme nitrate reductase. Other bacteria can reduce nitrate to nitrogen gas by also producing the enzyme nitrite reductase which reduces nitrite to nitrogen gas. Other organisms do not have the ability to reduce nitrate at all.
Nitrate Reduction Test
The nitrate reduction test determines if bacteria conduct nitrate reduction, and therefore if they have the gene for the nitrate reductase enzyme, resulting in the reduction of nitrate (NO3). To determine if nitrite is produce, nitrite (NO2) in the medium will form nitrous acid that reacts with the reagent sulfanilic acid (added to the medium), and that reacts with the another reagent naphthylamine (also added to the medium) to form a red color. To determine if denitrification occurs (N2 is produced), a Durham tube is used to capture the gas. If N2 or NO2 are not detected, rather than testing for ammonia (NH3) directly, zinc is used to determine if there is nitrate remaining in the medium. If there is nitrate remaining in the medium, this indicates that no nitrate reduction occurred and will eliminate the possibility that nitrate was reduced to ammonia. However, if there is no nitrate remaining and N2 or NO2 was not detected, this indicates that nitrate reduction occurred and ammonia was produced.
Interpretation of the Nitrate Reduction Test
Step 1: Examine Cultures for N2 Production
There are various ways that a bacterium can utilize nitrate as the final electron acceptor in anaerobic respiration. Some species will completely reduce nitrate to N2 gas, a process called denitrification. If denitrification occurred and therefore N2 was produced, there will be a pocket of gas (this is N2 gas) in the top of the Durham tube. The test tube is examined for the presence or absence of N2 in the Durham tube before any reagents are added.

Figure 1: Nitrate can be reduced to nitrogen gas (N2) by some bacterial species NO3 (nitrate) → NO2 (nitrite) → N2 (gas). If this occurs, there will be a bubble located inside of the Durham tube.
Step 2: Examine Cultures for NO2 Production
If there is no nitrogen gas (N2), it is still possible that nitrate (NO3) was reduced to nitrate (NO2). There are still a couple of possible outcomes that need examining to determine whether or not nitrate (NO3) was reduced:
- possibility 1: nitrate (NO3) reduction to nitrite (NO2)
- possibility 2: nitrate (NO3) reduction to ammonia (NH3)
- possibility 3: no reduction of nitrate (NO3)
To determine if nitrate was reduced to nitrate (possibility 1), reagents are added to the culture medium. A red color will be produced in the medium only when nitrite (NO2) is present in the medium, indicating nitrate reduction to nitrite.

Figure 2: Red coloration indicates that nitrate was reduced to produce nitrite NO3 (nitrate) → NO2 (nitrite).
Step 3: Examine Cultures for NH3 Production
Even if no N2 or NO2 were produced in the culture, it is still possible that nitrate was reduced in the culture. The following two possibilities still exist:
- possibility 2: nitrate (NO3) reduction to ammonia (NH3)
- possibility 3: no reduction of nitrate (NO3)
To differentiate between the above two possibilities, powdered zinc is added. The zinc will not show ammonia production. Instead, the zinc will show if the nitrate is still present in the medium. If the nitrate is still present in the medium, then the nitrate was not reduced and was therefore not reduced to ammonia (nitrate reduction negative).
After zinc is added to the medium, there are two possible outcomes:
- no pink color forms: this means that possibility 2 occurred and nitrate was reduced to ammonia.
- pink color forms: this means that possibility 3 occurred and no nitrate was reduced in the culture. If nitrate (NO3), is still be present in the tube, then zinc reduces the nitrate to nitrite, which then reacts with the 2 reagents already added to the tube producing a pink color.
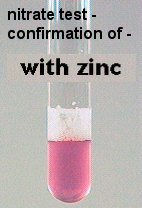
Figure 3: If no bubble is present in the Durham tube and the medium did not turn red, it is still possible that nitrate was reduced to ammonia. Zinc is added to determine whether the nitrate was reduced or not. If the nitrate was not reduced by the microbes in the tube, the zinc will cause the medium to turn pink and indicate that the bacteria are nitrate reduction negative. If the medium does not turn pink when zinc is added, this indicates that nitrate was reduced to ammonia by the microbe and is therefore nitrate reduction positive.
| REACTION | N2 gas | Color after adding reagents | Color after adding zinc |
|---|---|---|---|
| NO3 to NO2 | none | red | (Zn not added) |
| NO3 to N2 | yes | no color | (Zn not added) |
| NO3 to NH3 | none | no color | no color |
| no NO3 reduction | none | no color | pink-red |
Laboratory Instructions
- Inoculate the nitrate broths with the assigned bacterial species.
- Incubate at the optimal temperature, 30º C or 37º C, for these bacterial species.
- After Incubation: Look for N2 gas first before adding reagents and record results for whether or not N2 gas was produced.
- Add 6-8 drops of nitrite reagent A.
- Add the same number of drops of nitrite reagent B.
- You should see a reaction within a minute or less. Record results for whether or not nitrite was produced.
- If you have not seen evidence of either nitrite (NO2) or N2 gas, add a bit of powdered zinc to the culture medium. A bit of zinc is about the amount that sticks to the end of a wood stick.
- The reduction of unused nitrate (NO3) by zinc takes a couple of minutes. Record results for whether or not ammonia was produced.
Results & Questions
| NO3 present/absent (+/-) | NO2 present/absent (+/-) | N2 present/absent (+/-) | nitrate reduction (+/-) | |
|---|---|---|---|---|
| Alcaligenes faecalis | ||||
| Escherichia coli | ||||
| Pseudomonas aeruginosa |
- Complete the table above with experimental results from the nitrate reduction cultures.
- Name the 2 major end products of nitrate reduction.
- What does the gas pocket in the Durham tube indicate about nitrate reduction by that bacterial species? Be specific.
- What does the red color after adding reagents to the medium mean about nitrate reduction by that bacterial species? Be specific.
- What does a lack of pink color (no pink) after adding zinc mean about nitrate reduction by that bacterial species? Be specific.
- What does a pink color after adding zinc mean about nitrate reduction by that bacterial species? Be specific.
- Species that CAN conduct nitrate reduction may conduct the process differently. Explain this statement.
- Define denitrification.
- Is nitrate reduction an aerobic pathway or an anaerobic pathway?
Attributions
- General Microbiology Lab Manual (Pakpour & Horgan) by Nazzy Pakpour & Sharon Horgan is licensed under CC BY-SA 4.0
- Microbiology Labs I by Delmar Larsen and Jackie Reynolds has an undeclared license
- Red Mountain Microbiology by Jill Raymond Ph.D.; Graham Boorse, Ph.D.; and Anne Mason M.S is licensced under CC BY-NC 4.0


