14.3D: Telomere Replication
- Page ID
- 13294
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)As DNA polymerase alone cannot replicate the ends of chromosomes, telomerase aids in their replication and prevents chromosome degradation.
- Describe the role played by telomerase in replication of telomeres
Key Points
- DNA polymerase cannot replicate and repair DNA molecules at the ends of linear chromosomes.
- The ends of linear chromosomes, called telomeres, protect genes from getting deleted as cells continue to divide.
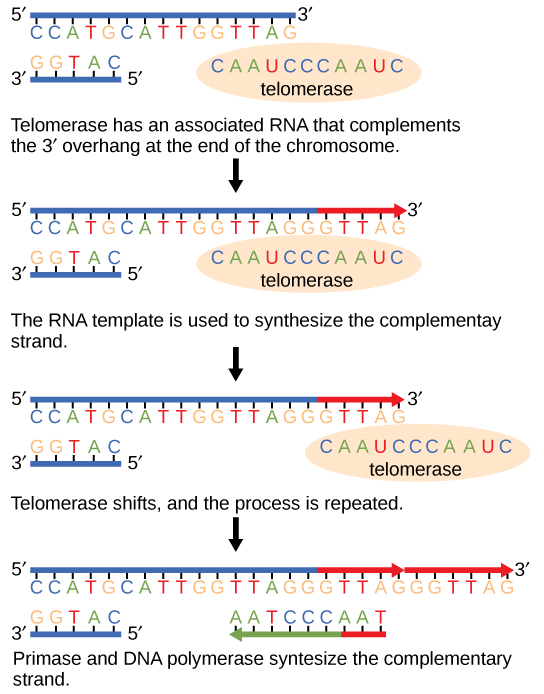
- The telomerase enzyme attaches to the end of the chromosome; complementary bases to the RNA template are added on the 3′ end of the DNA strand.
- Once the lagging strand is elongated by telomerase, DNA polymerase can add the complementary nucleotides to the ends of the chromosomes and the telomeres can finally be replicated.
- Cells that undergo cell division continue to have their telomeres shortened because most somatic cells do not make telomerase; telomere shortening is associated with aging.
- Telomerase reactivation in telomerase-deficient mice causes extension of telomeres; this may have potential for treating age-related diseases in humans.
Key Terms
- telomere: either of the repetitive nucleotide sequences at each end of a eukaryotic chromosome, which protect the chromosome from degradation
- telomerase: an enzyme in eukaryotic cells that adds a specific sequence of DNA to the telomeres of chromosomes after they divide, giving the chromosomes stability over time
The End Problem of Linear DNA Replication
Linear chromosomes have an end problem. After DNA replication, each newly synthesized DNA strand is shorter at its 5′ end than at the parental DNA strand’s 5′ end. This produces a 3′ overhang at one end (and one end only) of each daughter DNA strand, such that the two daughter DNAs have their 3′ overhangs at opposite ends

Every RNA primer synthesized during replication can be removed and replaced with DNA strands except the RNA primer at the 5′ end of the newly synthesized strand. This small section of RNA can only be removed, not replaced with DNA. Enzymes RNase H and FEN1 remove RNA primers, but DNA Polymerase will add new DNA only if the DNA Polymerase has an existing strand 5′ to it (“behind” it) to extend. However, there is no more DNA in the 5′ direction after the final RNA primer, so DNA polymerse cannot replace the RNA with DNA. Therefore, both daughter DNA strands have an incomplete 5′ strand with 3′ overhang.
In the absence of additional cellular processes, nucleases would digest these single-stranded 3′ overhangs. Each daughter DNA would become shorter than the parental DNA, and eventually entire DNA would be lost. To prevent this shortening, the ends of linear eukaryotic chromosomes have special structures called telomeres.
Telomere Replication
The ends of the linear chromosomes are known as telomeres: repetitive sequences that code for no particular gene. These telomeres protect the important genes from being deleted as cells divide and as DNA strands shorten during replication.
In humans, a six base pair sequence, TTAGGG, is repeated 100 to 1000 times. After each round of DNA replication, some telomeric sequences are lost at the 5′ end of the newly synthesized strand on each daughter DNA, but because these are noncoding sequences, their loss does not adversely affect the cell. However, even these sequences are not unlimited. After sufficient rounds of replication, all the telomeric repeats are lost, and the DNA risks losing coding sequences with subsequent rounds.
The discovery of the enzyme telomerase helped in the understanding of how chromosome ends are maintained. The telomerase enzyme attaches to the end of a chromosome and contains a catalytic part and a built-in RNA template. Telomerase adds complementary RNA bases to the 3′ end of the DNA strand. Once the 3′ end of the lagging strand template is sufficiently elongated, DNA polymerase adds the complementary nucleotides to the ends of the chromosomes; thus, the ends of the chromosomes are replicated.
Telomerase and Aging
Telomerase is typically active in germ cells and adult stem cells, but is not active in adult somatic cells. As a result, telomerase does not protect the DNA of adult somatic cells and their telomeres continually shorten as they undergo rounds of cell division.
In 2010, scientists found that telomerase can reverse some age-related conditions in mice. These findings may contribute to the future of regenerative medicine. In the studies, the scientists used telomerase-deficient mice with tissue atrophy, stem cell depletion, organ failure, and impaired tissue injury responses. Telomerase reactivation in these mice caused extension of telomeres, reduced DNA damage, reversed neurodegeneration, and improved the function of the testes, spleen, and intestines. Thus, telomere reactivation may have potential for treating age-related diseases in humans.
Contributions and Attributions
- OpenStax College, Biology. October 22, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44487/latest...ol11448/latest. License: CC BY: Attribution
- OpenStax College, Biology. October 29, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44487/latest...ol11448/latest. License: CC BY: Attribution
- DNA replication. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/DNA%20replication. License: CC BY-SA: Attribution-ShareAlike
- isotope. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/isotope. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, Biology. October 29, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44487/latest...ol11448/latest. License: CC BY: Attribution
- OpenStax College, Biology. October 29, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44487/latest...ol11448/latest. License: CC BY: Attribution
- OpenStax College, Biology. November 2, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44488/latest...ol11448/latest. License: CC BY: Attribution
- DNA replication. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/DNA%20replication. License: CC BY-SA: Attribution-ShareAlike
- origin of replication. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/origin%...%20replication. License: CC BY-SA: Attribution-ShareAlike
- helicase. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/helicase. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, Biology. October 29, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44487/latest...ol11448/latest. License: CC BY: Attribution
- OpenStax College, Biology. October 29, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44487/latest...ol11448/latest. License: CC BY: Attribution
- OpenStax College, DNA Replication in Prokaryotes. November 2, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44488/latest/#tab-ch14_04_01. License: CC BY: Attribution
- Many enzymes are involved in the DNA replication fork.. Provided by: Wikimedia Commons. Located at: en.Wikipedia.org/wiki/DNA_rep...ication_en.svg. License: Public Domain: No Known Copyright
- origin of replication. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/origin%...%20replication. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, DNA Replication. October 29, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m45475/latest/. License: CC BY: Attribution
- leading strand. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/leading%20strand. License: CC BY-SA: Attribution-ShareAlike
- lagging strand. Provided by: Wikipedia. Located at: en.Wikipedia.org/wiki/lagging%20strand. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, Biology. October 29, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44487/latest...ol11448/latest. License: CC BY: Attribution
- OpenStax College, Biology. October 29, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44487/latest...ol11448/latest. License: CC BY: Attribution
- OpenStax College, DNA Replication in Prokaryotes. November 2, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44488/latest/#tab-ch14_04_01. License: CC BY: Attribution
- Many enzymes are involved in the DNA replication fork.. Provided by: Wikimedia Commons. Located at: en.Wikipedia.org/wiki/DNA_rep...ication_en.svg. License: Public Domain: No Known Copyright
- DNA Replication. Located at: http://www.youtube.com/watch?v=4jtmOZaIvS0. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- OpenStax College, DNA Replication. October 29, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m45475/latest/. License: CC BY: Attribution
- OpenStax College, Biology. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44517/latest...ol11448/latest. License: CC BY: Attribution
- telomere. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/telomere. License: CC BY-SA: Attribution-ShareAlike
- telomerase. Provided by: Wiktionary. Located at: en.wiktionary.org/wiki/telomerase. License: CC BY-SA: Attribution-ShareAlike
- OpenStax College, Biology. October 29, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44487/latest...ol11448/latest. License: CC BY: Attribution
- OpenStax College, Biology. October 29, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44487/latest...ol11448/latest. License: CC BY: Attribution
- OpenStax College, DNA Replication in Prokaryotes. November 2, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44488/latest/#tab-ch14_04_01. License: CC BY: Attribution
- Many enzymes are involved in the DNA replication fork.. Provided by: Wikimedia Commons. Located at: en.Wikipedia.org/wiki/DNA_rep...ication_en.svg. License: Public Domain: No Known Copyright
- DNA Replication. Located at: http://www.youtube.com/watch?v=4jtmOZaIvS0. License: Public Domain: No Known Copyright. License Terms: Standard YouTube license
- OpenStax College, DNA Replication. October 29, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m45475/latest/. License: CC BY: Attribution
- OpenStax College, DNA Replication in Eukaryotes. October 16, 2013. Provided by: OpenStax CNX. Located at: http://cnx.org/content/m44517/latest...e_14_05_01.jpg. License: CC BY: Attribution


