20.2: Air Pollution
- Page ID
- 47005
Air pollution occurs in many forms but can generally be thought of as gaseous and particulate contaminants that are present in the earth’s atmosphere. Chemicals discharged into the air that have a direct impact on the environment are called primary pollutants. These primary pollutants sometimes react with other chemicals in the air to produce secondary pollutants.
Air pollution is typically separated into two categories: outdoor air pollution and indoor air pollution. Outdoor air pollution involves exposures that take place outside of the built environment. Examples include fine particles produced by the burning of coal, noxious gases such as sulfur dioxide, nitrogen oxides and carbon monoxide; ground-level ozone and tobacco smoke. Indoor air pollution involves exposures to particulates, carbon oxides, and other pollutants carried by indoor air or dust. Examples include household products and chemicals, out-gassing of building materials, allergens (cockroach and mouse dropping, mold, pollen), and tobacco smoke.
This video summarizes the causes, consequences, and solutions to air pollution.
Sources of Air Pollution
A stationary source of air pollution refers to an emission source that does not move, also known as a point source. Stationary sources include factories, power plants, and dry cleaners. The term area source is used to describe many small sources of air pollution located together whose individual emissions may be below thresholds of concern, but whose collective emissions can be significant. Residential wood burners are a good example of a small source, but when combined with many other small sources, they can contribute to local and regional air pollution levels. Area sources can also be thought of as non-point sources, such as construction of housing developments, dry lake beds, and landfills.
A mobile source of air pollution refers to a source that is capable of moving under its own power. In general, mobile sources imply “on-road” transportation, which includes vehicles such as cars, sport utility vehicles, and buses. In addition, there is also a “non-road” or “off-road” category that includes gas-powered lawn tools and mowers, farm and construction equipment, recreational vehicles, boats, planes, and trains.
Agricultural sources arise from operations that raise animals and grow crops, which can generate emissions of gases and particulate matter. For example, animals confined to a barn or restricted area produce large amounts of manure. Manure emits various gases, particularly ammonia into the air. This ammonia can be emitted from the animal houses, manure storage areas, or from the land after the manure is applied. In crop production, the misapplication of fertilizers, herbicides, and pesticides can potentially result in aerial drift of these materials and harm may be caused.
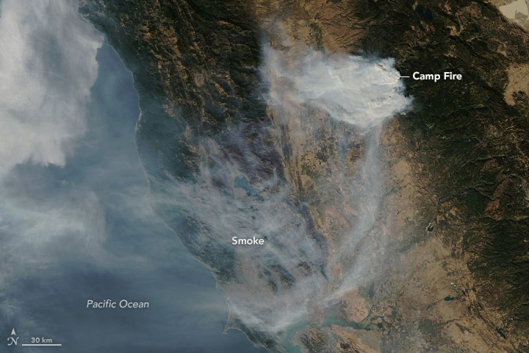
Unlike the above mentioned sources of air pollution, air pollution caused by natural sources is not caused by people or their activities. An erupting volcano emits particulate matter and gases, forest and prairie fires can emit large quantities of pollutants (figure \(\PageIndex{a}\)), dust storms can create large amounts of particulate matter, and plants and trees naturally emit volatile organic compounds which can form aerosols that can cause a natural blue haze. Wild animals in their natural habitat are also considered natural sources of “pollution”.
Criteria Air Pollutants
The Environmental Protection Agency has identified six common air pollutants called criteria air pollutants. They are particulate matter, ground-level ozone, carbon monoxide, sulfur oxides, nitrogen oxides, and lead. These pollutants can harm health and the environment, and cause property damage. Of the six pollutants, particle pollution and ground-level ozone are the most widespread health threats. The U.S. Environmental Protection Agency (EPA) regulates them by developing criteria based on considerations of human and environmental health.
- Ground-level ozone is not emitted directly into the air, but is created by chemical reactions between nitrogen oxides (NOX) and the gaseous oxygen (O2) in the atmosphere react in the presence of sunlight. It also forms when nitrogen oxides and volatile organic compounds (VOCs) react. Emissions from industrial facilities and electric utilities, motor vehicle exhaust, gasoline vapors, and chemical solvents are some of the major sources of NOX and VOCs. Breathing ozone can trigger a variety of health problems, particularly for children, the elderly, and people of all ages who have lung diseases such as asthma. Ground level ozone can also have harmful effects on sensitive vegetation and ecosystems. (Ground-level ozone should not be confused with the ozone layer, which is high in the atmosphere and protects Earth from ultraviolet light; ground-level ozone provides no such protection).
- Particulate matter, also known as particle pollution, is a complex mixture of extremely small particles and liquid droplets. Particle pollution is made up of a number of components, including acids (such as nitrates and sulfates), organic chemicals, metals, and soil or dust particles. Examples of human-generated particulate matter are from combustion (such as burning wood or coal) and mining (which releases dust). Wildfires or wind erosion are natural sources of particulate matter. The size of particles is directly linked to their potential for causing health problems. EPA is concerned about particles that are 10 micrometers in diameter or smaller because those are the particles that generally pass through the throat and nose and enter the lungs. Furthermore, particles that are 2.5 micrometers in diameter or smaller can pass from the lungs into the bloodstream, through which they are transported to various organs and cause serious health effects.
- Carbon monoxide (CO) is a colorless, odorless gas emitted from combustion processes. Nationally and, particularly in urban areas, the majority of CO emissions to ambient air come from vehicles. However, volcanic eruptions and wildfires also release carbon monoxide. Carbon monoxide can cause harmful health effects by reducing oxygen delivery to the body’s organs (like the heart and brain) and tissues. At extremely high levels, CO can cause death.
- Nitrogen oxides (NOX) are a group of highly reactive gasses, which includes nitrogen monoxide (NO) and nitrogen dioxide (NO2). EPA’s National Ambient Air Quality Standard uses NO2 as the indicator for the larger group of nitrogen oxides. Nitrogen oxides forms quickly from emissions from cars, trucks and buses, power plants, and off-road equipment. Additionally, nitrogen oxides are released naturally by certain bacteria. In addition to contributing to the formation of ground-level ozone, and fine particle pollution, nitrogen oxides react in the atmosphere to form nitric acid (HNO3) and nitrous acid (HNO2), which are components of Acid Deposition. Nitrogen oxides is linked with a number of adverse effects on the respiratory system.
- Sulfur dioxide (SO2) is mainly released from fossil fuel combustion at power plants (73%) and other industrial facilities (20%). Smaller sources of SO2 emissions include industrial processes such as extracting metal from ore, and the burning of high sulfur containing fuels by locomotives, large ships, and non-road equipment. Volcanoes are a natural source of SO2. Sulfur dioxide is linked with a number of adverse effects on the respiratory system. It reacts in the atmosphere to form sulfuric acid (H2SO4), a component of Acid Deposition.
- Lead is a metal found naturally in the environment as well as in manufactured products. The major sources of lead emissions have historically been from fuels in on-road motor vehicles (such as cars and trucks) and industrial sources. As a result of regulatory efforts in the U.S. to remove lead from on-road motor vehicle gasoline, emissions of lead from the transportation sector dramatically declined by 95 percent between 1980 and 1999, and levels of lead in the air decreased by 94 percent between 1980 and 1999. Today, the highest levels of lead in air are usually found near lead smelters. The major sources of lead emissions to the air today are ore and metals processing and piston-engine aircraft operating on leaded aviation gasoline. Volcanoes and erosion are natural sources of lead.
Indoor Air Pollution (Major concerns in developed countries)
Most people spend approximately 90 percent of their time indoors. However, the indoor air we breathe in homes and other buildings can be more polluted than outdoor air and can increase the risk of illness. There are many sources of indoor air pollution in homes. They include biological contaminants such as bacteria, molds and pollen, burning of fuels and environmental tobacco smoke, building materials and furnishings, household products, central heating and cooling systems, and outdoor sources. Outdoor air pollution can enter buildings and become a source of indoor air pollution.
Sick building syndrome is a term used to describe situations in which building occupants have health symptoms that are associated only with spending time in that building. Causes of sick building syndrome are believed to include inadequate ventilation, indoor air pollution, and biological contaminants. Usually indoor air quality problems only cause discomfort. Most people feel better as soon as they remove the source of the pollution. Making sure that your building is well-ventilated and getting rid of pollutants can improve the quality of your indoor air.
Secondhand Smoke (Environmental Tobacco Smoke)
Secondhand smoke is the combination of smoke that comes from a cigarette and smoke breathed out by a smoker. When a non-smoker is around someone smoking, they breathe in secondhand smoke.
Secondhand smoke is dangerous to anyone who breathes it in. There is no safe amount of secondhand smoke. It contains over 7,000 harmful chemicals, at least 250 of which are known to damage human health. It can also stay in the air for several hours after somebody smokes. Even breathing secondhand smoke for a short amount of time can hurt your body.
Over time, secondhand smoke can cause serious health issues in non-smokers. The only way to fully protect non-smokers from the dangers of secondhand smoke is to not allow smoking indoors. Separating smokers from nonsmokers (like “no smoking” sections in restaurants)‚ cleaning the air‚ and airing out buildings does not completely get rid of secondhand smoke.
Source: Smokefree.gov
Addressing Air Pollution
A combination of regulatory, economic, and technological strategies can reduce air pollution. The 1970 Clean Air Act is a key federal regulation that has successfully reduced emissions of air pollutants such as lead and sulfur dioxide. The Environmental Protection Agency sets an air quality standard for large emitters of criteria air pollutants, which is the maximum allowable amount of the pollutant that can be released. Each state then develops a plan to comply with these standards. Note that hundreds of air pollutants beyond the six criteria air pollutants are regulated under the Clean Air Act.
Economic incentives make it financially beneficial for individuals, institutions, or companies to use technologies or otherwise take action that limits air pollution. For examples, green taxes increase the cost of an environmentally harmful action. For example, Canada levies a green tax on fuel-inefficient vehicles. Subsidies, on the other hand reduce the cost of environmentally beneficial choices. For example, the federal solar tax credit in the United States allows homeowners to claim a percentage of the cost of installing solar panels when their filing taxes This amount would thus reduce taxes owed or be issued as a tax refund. (Increased usage of solar electricity ultimately reduces the need for coal and natural gas power plants, which generate air pollution.)
Tradable permits (cap-and trade) is another economic incentive. First, the total allowable amount of emissions for an entity (such as a state or country) is set. Next, emission credits are distributed to potential polluters. Industries that reduce emissions can sell their credits, while those with high emissions may be required. For example, large emitters of greenhouse gases in California must by carbon pollution permits. The purchase of these permits funds the California Climate Credit, which Californians receive as a reduction to their April and October electricity bills.
Finally, a variety of technologies reduce air pollution. For example, air filters and proper ventilation are essential for limiting indoor air pollution. Advances in public transportation (and policies that promote them), and energy-efficient vehicles further reduce air pollution. Electric vehicles also reduce air pollution (figure \(\PageIndex{b}\)), particularly if the the electricity that supplies them comes from a clean, renewable source, such as solar panels. Catalytic converters make emissions from standard vehicles less harmful by facilitating chemical reactions. For example, they catalyze reactions that convert nitrogen oxides (NOX) to nitrogen gas (N2) and carbon monoxide (CO) to carbon dioxide (CO2). (While carbon dioxide is a greenhouse gas, it is not directly poisonous as carbon monoxide is.)
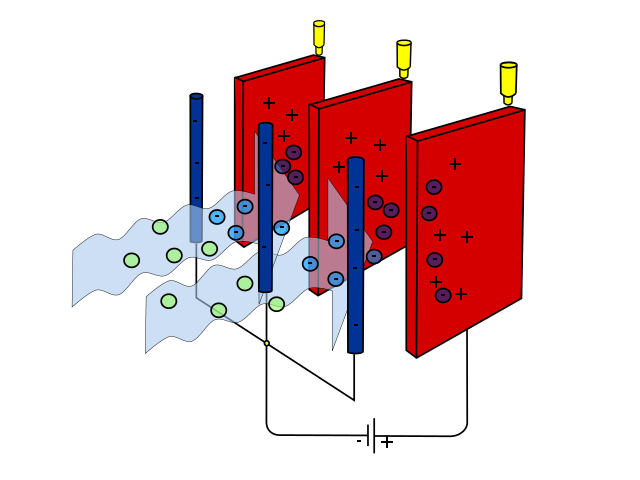
Other technologies limit emissions from industry. For example, smokestack scrubbers remove some pollutants from power plant emissions before they are released and have reduced sulfur dioxide emissions from burning coal. Electrostatic precipitators negatively charge pollutants and remove charged pollutants with a positive electrode (figure \(\PageIndex{c}\)). While electrostatic precipitators were originally developed for industry, smaller versions can be used as air purifiers in homes or businesses.

Attribution
Modified by Melissa Ha from the following sources:
- Atmospheric Pollution from Environmental Biology by Matthew R. Fisher (licensed under CC-BY)
- Basic Information about NO2. Environmental Protection Agency. Accessed 11-04-2021 (public domain).


