4.4.7: Other Signaling Molecules
- Page ID
- 32031
Learning Objectives
- Identify several signaling molecules beyond the five major plant hormones and describe their effects.
- Distinguish between the hypersensitive response and systemic acquired response.
- Explain the mechanisms by which signaling compounds aid in plant defense against pathogens and herbivores.
Recent research has discovered a number of compounds that also influence plant development. Their roles are less understood than the effects of the major hormones described so far.
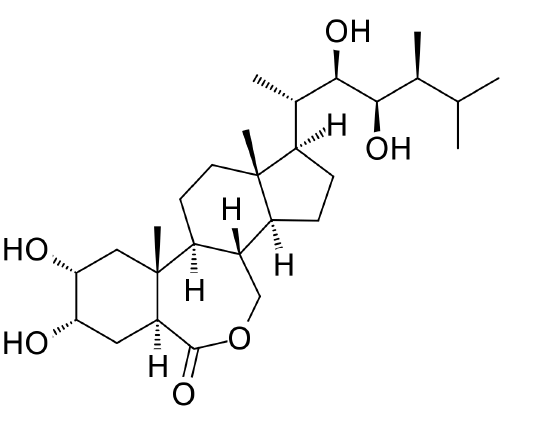
Brassinosteroids
Brassinosteroids (Figure \(\PageIndex{1}\)) are synthesized primarily in young tissues are important to many developmental and physiological processes. In fact, many sources considere them the sixth major plant hormones. Unlike the hormones discussed previously, brassinosteroids do not travel far from their site of synthesis. Signals between these compounds and other hormones, notably auxin and GAs, amplifies their physiological effect. Apical dominance, seed germination, gravitropism, lateral root formation, differentiation of cells in the vascular tissue, and resistance to freezing are all positively influenced by brassinosteroids. Root growth and fruit dropping are inhibited by steroids.
Systemin
Systemin, named for the fact that it is distributed systemically (everywhere) in the plant body upon production, is a short polypeptide that activates plant responses to wounds from herbivores (animals that feed on plant parts). It causes the plant to produce jasmonic acid (see below).
Jasmonates
Jasmonates play a major role in defense responses to herbivory (Figure \(\PageIndex{2}\)). Their levels increase when a plant is wounded by an herbivore, resulting in an increase in toxic secondary metabolites. For example, jasmonic acid (Figure \(\PageIndex{3}\)) also induces transcription of protease inhibitors. Protease inhibitors both taste bad and prevent breakdown of proteins in the herbivore’s gut, thus making the insect sick and deterring further herbivory. Jasmonates also contribute to the production of volatile compounds that attract natural enemies of herbivores. Chewing of tomato plants by caterpillars leads to an increase in jasmonic acid levels, which in turn triggers the release of volatile compounds that attract predators of the pest. Jasmonates also elicit the synthesis of volatile compounds that attract parasitoids, which are insects that spend their developing stages in or on another insect, and eventually kill their host.

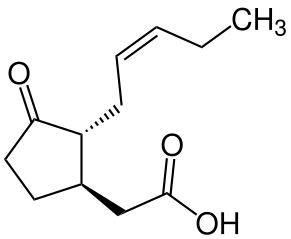
Jasmonates also work with systemin to mediate responses to drought, damage by ground-level ozone, and ultraviolet light.
Salicylic Acid
Salicylic acid resembles aspirin (Figure \(\PageIndex{4}\)) and is important for plant defense. It initiates the a systemic (whole body) response called the systemic acquired response (SAR) as a response to infection by parasites or pathogens. When a parasite or pathogen infects a cell, there is an specific, localized response called the hypersensitive response (HR). Following this very localized response, the plant initiates a systemic (whole body) response called the systemic acquired response (SAR). Salicylic acid is produced and converted to methyl salicylate (Figure \(\PageIndex{4}\)) inducing the SAR in response to the HR. The SAR activates transcription of general “pathogenesis-resistance” genes, which are not pathogen-specific (unlike in the hypersensitive response), but serve as general defense against pathogenic infection. The SAR is slower than the hypersensitive response, and also differs in that it is systemic instead of localized to the site of the infection.
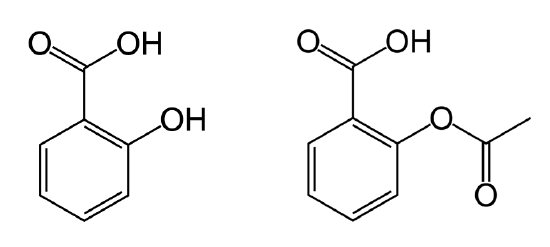
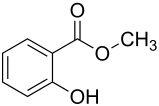
Similar to jasmonic acid, salicylic acid can mediates defense against insect herbivores. It is directly toxic to some herbivores. Additionally, in response to herbivory, salicylic acid can be converted to methyl salicylate, which is released as a gas. This volatile compound can attract natural predators and parasites of the herbivores.
Some plants, such as skunk cabbage (Figure \(\PageIndex{5}\)) and elephant yam, are adapted to flower while snow still covers the ground. Salicylic acid mediates their ability to produce heat to melt the snow around them. Such plants are thus called thermogenic ("heat producing").

Oligosaccharins
Oligosaccharins are short chains of simple sugars that play a role in plant defense against bacterial and fungal infections. They act locally at the site of injury, and can also be transported to other tissues.
Strigolactones
Strigolactones (Figure \(\PageIndex{6}\)) promote seed germination in some species and inhibit lateral apical development in the absence of auxins. Strigolactones also play a role in the establishment of mycorrhizae, a mutualistic association of plant roots and fungi.
Florigen
Florigen is a systemic signal that initiates flowering. It is also involved in the formation of storage organs and contributes to plant architecture. It is synthesized in leaves and transported to the shoot apical meristem (SAM) where it promotes flowering in response to daylength cues. At the molecular level, florigen is represented as a protein product encoded by the FLOWERING LOCUS T (FT) gene, which is highly conserved (occurs/has a similar genetic sequence in) across flowering plants.
Florigen is considered one of the important targets for crop improvement. Regulation of flowering time is an important target for plant breeding because the control of flowering to a favorable time provides successful grain production in a given cropping area. Flowering at unfavorable seasons causes loss of yield due to insufficient growth of photosynthetic organs or poor fertility due to heat or cold stress during reproduction. Thus, understanding florigen function can contribute to novel breeding techniques in crops to produce cultivars that can start their reproductive stage at optimal seasons.
Supplemental Reading
Filgueiras, C. C., Martins, A. D., Pereira, R. V., & Willett, D. S. (2019). The Ecology of Salicylic Acid Signaling: Primary, Secondary and Tertiary Effects with Applications in Agriculture. International journal of molecular sciences, 20 (23), 5851. https://doi.org/10.3390/ijms20235851
Attributions
Curated and authored by Melissa Ha from the following sources:
- 30.6 Plant Sensory Systems and Responses from Biology 2e by OpenStax (licensed CC-BY). Access for free at openstax.org.
- Plant Hormones and Sensory Systems by Biology 1520 Introduction to Organismal Biology (licensed CC BY-NC-SA)
- Tsuji H. (2017). Molecular function of florigen. Breeding science, 67 (4), 327–332. https://doi.org/10.1270/jsbbs.17026 (licensed CC BY)


