10.1: FDA Monitoring and Enforcement
- Page ID
- 39533
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Over the last few chapters, you have explored the FDA organization, the Centers overseeing different products, and dove into some of the product areas themselves. Next, we will examine the regulations that govern this process and how the FDA inspects and enforces laws in this area to protect public health.
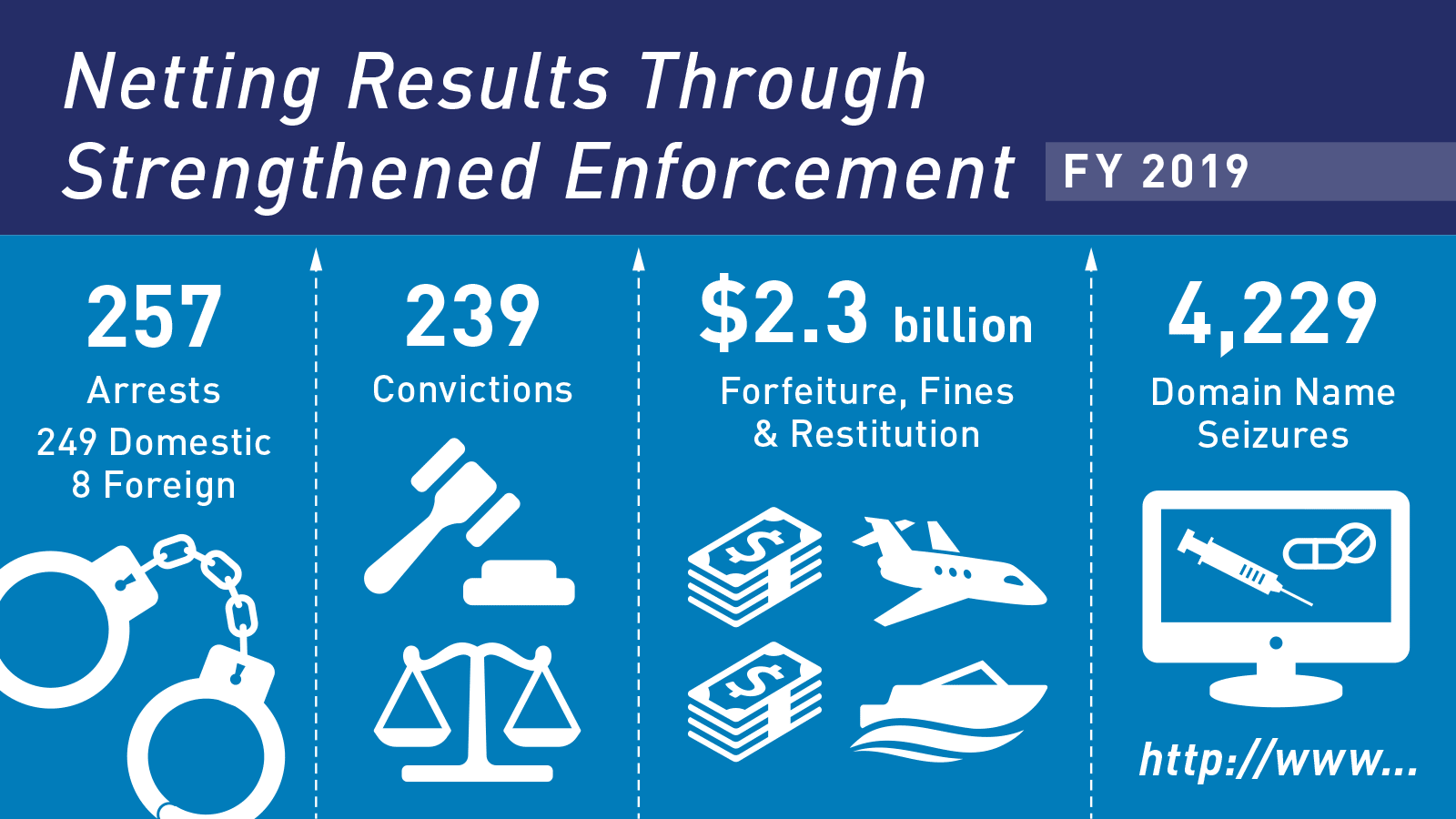
The FDA is mandated by the FD&C Act to protect public health from adulterated and misbranded regulated products and has significant and broad enforcement power to do so. The FD&C Act is the basic food and drug law of the U.S. With numerous amendments, it is the most extensive law of its kind in the world. The law is intended to assure the consumer that foods are pure and wholesome, safe to eat, and produced under sanitary conditions; that drugs and devices are safe and effective for their intended uses; that cosmetics are safe and made from appropriate ingredients; and that all labeling and packaging is truthful, informative, and not deceptive.
Companies are highly encouraged to engage the FDA in all forms of communication as these communication methods serve as prior notice of a lawsuit. However, aside from lawsuits, or regulations where the FDA has recall authority, most communication responses from the company are voluntary.
Explore!
Go to the following website and read the statement by the acting commissioner, Dr. Ned Sharples, regarding global enforcement strategies. Consider, why do we care about global enforcement? https://www.fda.gov/news-events/fda-voices-perspectives-fda-leadership-and-experts/expanding-criminal-enforcement-operations-globally-protect-public-health
Inspections, Compliance, Enforcement, and Criminal Investigations
As FDA’s criminal law enforcement arm, the Office of Criminal Investigations (OCI) protects the American public by conducting criminal investigations of illegal activities involving FDA-regulated products, arresting those responsible, and bringing them before the Department of Justice for prosecution. https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/criminal-investigations
Since 1993, OCI has investigated thousands of criminal schemes involving a broad range of criminal conduct, including the distribution of foreign counterfeit, unapproved, and misbranded medical products and clinical investigators and health fraud involving FDA-regulated drugs and medical devices.
Explore!
Explore some of the examples of the types of criminal investigations OCI investigates. Did you discover anything interesting?
- Cyber Crime
- Prescription Drugs
- Foods, Dietary Supplements
- Medical Devices
- Tobacco
- Veterinary Drugs
- Most Wanted Fugitives
Compliance Programs: www.fda.gov/inspections-compliance-enforcement-and-criminalinvestigations/compliance-manuals/compliance-program-guidance-manual-cpgm
Compliance Activities: www.fda.gov/inspections-compliance-enforcement-and-criminalinvestigations/compliance-actions-and-activities
Inspection Activities: www.fda.gov/inspections-compliance-enforcement-and-criminalinvestigations/inspection-references
FD&C Act Section 331
The list of prohibited acts in which the FDA may pursue action against a company for is outlined in the FD&C Act Section 331. These all apply if the product is regulated by the FDA; biologics, drugs, devices, cosmetics. Below are some key areas as they relate to enforcement. You will notice that it makes companies responsible for adulteration, even for outside third-party vendors.
Common Violations of the FD&C Act
- Adulterated or misbranded regulated product
- Receiving or delivering an adulterated or misbranded product
- Refusal for inspection
- Counterfeiting product
- Altering a product label
- Not registering a product
- Refusal to provide required documents
Code of Federal Regulations for IND, NDA, ANDA, and BLA
Explore!
FDA has the authority to not only enforce the laws enacted by the U.S. Congress but also communicate regulations in the Code of Federal Regulations under Title 21. The following Code of Federal Regulations(CFR) sections provide regulations for INDs, NDAs, and BLAs. All parts of section 21 of the Code of Federal Regulations are also available. To view the most current CFRs, go to https://www.ecfr.gov, and select Title 21 in the pull-down and GO. Use the “simple search” to complete the table below.
| CFR Sections | |
|---|---|
| BLA | Part 600-Biological Products: General Part 601-Licensing Part 610-General Biological Products Standards |
| IND | |
| NDA | |
| ANDA | |
| IND |


