14.1: Introduction
- Page ID
- 24902
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Genetic Manipulation (Selection)
Genetic modification of organisms has been occurring through human manipulation since the beginning of agriculture. Humans selectively bred crops and livestock to propagate desirable traits in a process termed artificial selection. The original grass that gave rise to domesticated corn called teosinte hardly resembles what we think of when imagining modern maize.

Teosinte, the progenitor of maize. Corn came about due to selective breeding. Credit: John Doebley (CC-BY)
Variation: Crop Domestication
Selective breeding can yield a variety of features even within the same species. Below is a selection of vegetables of the species Brassica oleracea that have been developed into different varieties over the course of agricultural history.

Cabbage: Brassica oleracea var. capitata Credit: Forest & Kim Starr (CC-BY 3.0)

Broccoli: Brassica oleracea var. italica Credit: Coyau (CC-BY-SA 3.0)

Kohlrabi: Brassica oleracea var. gongylodes Coyau (CC-BY-SA 3.0)

Romanesco: Brassica oleracea var. botrytis Credit: Richard Bartz (CC-BY-SA 2.5)
Variation: Animal Domestication

Credit: Mary Bloom, American Kennel Club (CC-BY-SA 4.0)

Companion animals like dogs underwent thousands of years of domestication and selection for traits that were desirable for different circumstances. A high degree of morphological diversity exists between dog breeds and their ancestral grey wolf progenitor.
Genetic Manipulation (Engineered)
Artificial selection takes multiple generations over a long period of time. With the advent of recombinant DNA and biotechnology, scientists can now genetically modify organisms through the introduction of foreign genes to provide desirable characteristics within one generation. This process does not require traits to naturally arise in a species.


GloFish are transgenic zebrafish (Danio rerio) expressing variants of GFP. Bottom features a wild-type fish. Credit: Azul (CC-BY)
GloFish® are novelty pets that have the insertion of various cnidarian fluorescent protein genes into the genome. These fish were released in the United States in 2003 and have subsequently been developed in red, orange, and blue varieties. Black tetras and tiger barbs are also now available.

Black tetra (Gymnocorymbus ternetzi) GloFish Credit: https://www.flickr.com/photos/fergy08/ (CC-BY 2.0)
Wild-type Black Tetra Credit: Fernandograu (CC-BY-SA 3.0)
Genetic Engineering in Plants
With the advent of agribusiness, agriculture has become a profit-driven venture independent of food production. In this case, high production is paramount. Whereas traditional agriculture or artificial selection was slow and methodical, genetic modification in the context of agribusiness is instantaneous through genetic engineering. The objective of genetic engineering is to transfer the DNA encoding a useful or favorable gene from an organism that carries that gene to one that does not. Simply inserting DNA into an organism does not result in expression. An appropriate promoter for the transgenic organism must be upstream of the gene of interest in order to drive transcription. In mammals, a strong promoter that will result in expression in every cell is the CMV promoter that is derived from cytomegalovirus. Likewise in plants, a strong promoter that works in every cell is derived from viral promoters like the CaMV promoter from cauliflower mosaic virus from the 35S gene (a ribosomal RNA). (CaMV 35S sequence on NCBI)
Examples of useful traits include:
- Degrade herbicides
- Kill agricultural pests
- Synthesize critical nutrients
- To improve color and taste
- Resist damage during transit or prolonged storage.
- Increase in size
- Reduce time to market (more rapid growth or maturation)
Genetically Modified foods have become a hot topic of contention in recent times. These crops are generated through the infection of plant cells by a bacterium called Agrobacterium tumefaciens. Agrobacterium is a gram-negative alphaproteobacterium of the family Rhizobiaceae which includes symbiotic nitrogen fixers found in legumes. Unlike those symbionts, Agrobacterium is a pathogenic soil bacterium known as a causative agent of crown galls (tumors).

Crown gall on a Kalanchoë infected with Agrobacterium tumefaciens Credit: Bhai (CC-BY-SA 3.0)
The tumors are caused by the infection of plant cells by the bacterium and the subsequent insertion of the T-DNA (“Transfer DNA”) that has a tumor inducing capability (Ti). Through the engineering of the T-DNA in a plasmid, selected genes can be delivered to plant cells through infection of transformed bacteria.
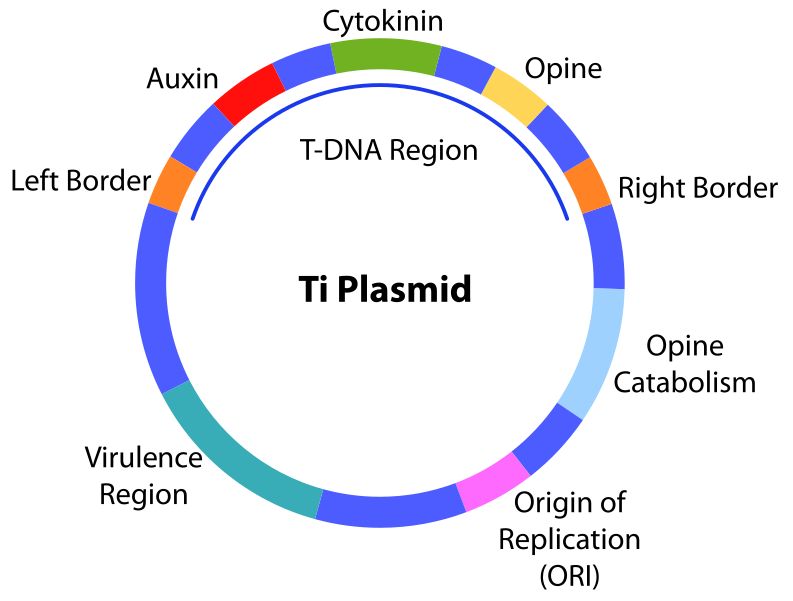
Ti plasmid has the T-DNA region replaced by the transgene. Ti is transformed into Agrobacterium which transduces the DNA into plant cells.
A modified Ti plasmid called pGreen was engineered to provide an MCS and selection marker for insertion of foreign genes of interest. In order for these genes to be expressed, they are driven by strong plant promoters like those from the CaMV 35S gene.

The plant to be engineered is cultured and infected with the transformed Agrobacteria that will then induce cysts that eventually root. The strong promoter of the CaMV 35S will constitutively express the gene in all cells of the plant.
Transformation of wild potato in culture using agrobacterium Credit: Seb951 (CC-BY-SA 3.0)
Growth of GMO Crops
Land area used in millions of hectares.
Damage Resistance
The first genetically modified crop FDA approved for sale was known as the Flavr Savr tomato. Tomatoes are prone to damage during shipping and are therefore picked before ripening. However, vine-ripened tomatoes have a richer flavor. Calgene developed the Flavr Savr and sold it to market between 1994-1997 in the United States. Flavr Savr was modified by inserting antisense into the genome that knocked-down the expression of polygalacturonase. Polygalacturonase degrades pectin in the cell walls of the fruit that results in softening, proclivity to damage, and eventual rotting.
Herbicide Resistance

Roundup is the trade-name of the herbicide glyphosate used in agriculture to control weed populations developed and patented by Monsanto. Glyphosate is absorbed through the foliage of plants and interferes with the enzymes that aid in the production of tyrosine, phenylalanine, and tryptophan. Plants and lower organisms generate aromatic amino acids through an enzyme 5-enolpyruvylshikimate-3-phosphate (EPSP) synthase which is the target of this compound. A series of Roundup Ready crops were designed by the insertion of the Agrobacterium EPSP gene driven by the CaMV 35S promoter. This version of the gene is inherently resistant to glyphosate poisoning.
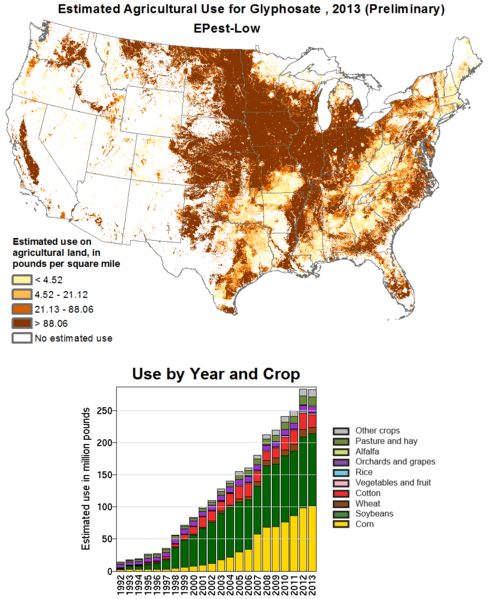
The Emergence of Superweeds

Palmer amaranth (Amaranthus palmeri), commonly referred to as pigweed, is a pest species in cotton and soy fields that have become glyphosate-resistant.
Credit: Pompilid (CC-BY-SA 3.0)
Pest Resistance

Credit: Cyndy Sims Parr (CC-BY-SA)
Crystals from the Bacillus thuringiensis (Bt), called Cry protein, are toxic to various insects: moths & butterflies, beetles, ants, wasps, flies & mosquitoes, bees, nematodes. The insertion of this gene into plants like corn (Bt-Corn) was designed to be resistant to pests.

Disease Resistance
Papayas in the United States primarily come from Hawaii. A virus known as Papaya Ringspot Disease threatened the papaya crop in Hawaii. To combat this, papayas were genetically engineered to block viral entry into the papaya cells. Papayas purchased in Latin markets are most likely unmodified and usually come from Mexico where the ringspot disease is not yet a problem.

Plum Pox Virus is a threat to the genus Prunus. A genetically modified plum plant has been developed called C5. The cells in these plants silence the expression of plum pox coat protein if infected to block propagation of the virus.

An apricot infected with plum pox.

C5 resistant plums
Nutritional Engineering
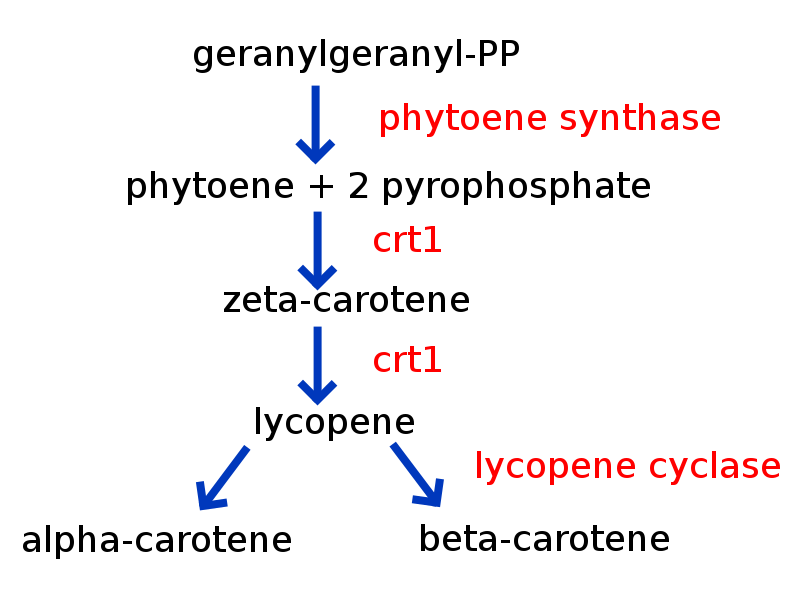
Credit: en:user: Petaholmes, (CC-BY-SA 3.0)
Golden rice is a genetically engineered rice that is meant to address Vitamin A deficiency. It introduces enzyme genes from other species involved in the biosynthetic pathways for β-carotene production, a vitamin A precursor. It is estimated that millions of deaths and irreversible blindness occur in the third world each year due to Vitamin A deficiency and creating this rice was meant to address the problem.
Rice is a staple in many cultures, therefore it is a good delivery system. Many controversies exist surrounding Golden Rice due to anti-GMO sentiment (from patenting systems), cultural sensitivities (white rice revered in certain cultures), and Vitamin A content/conversion doubts.

Explore the Debate
- Case studies reveal the complex truths behind GM crop myths.
- Case studies: A hard look at GM crops. Gilbert N. Nature. 2013 May 2;497(7447):24-6. doi: 10.1038/497024a
Heterologous Expression in Tissue Culture
Human Embryonic Kidney cells (HEK293T) expressing the green fluorescent protein.
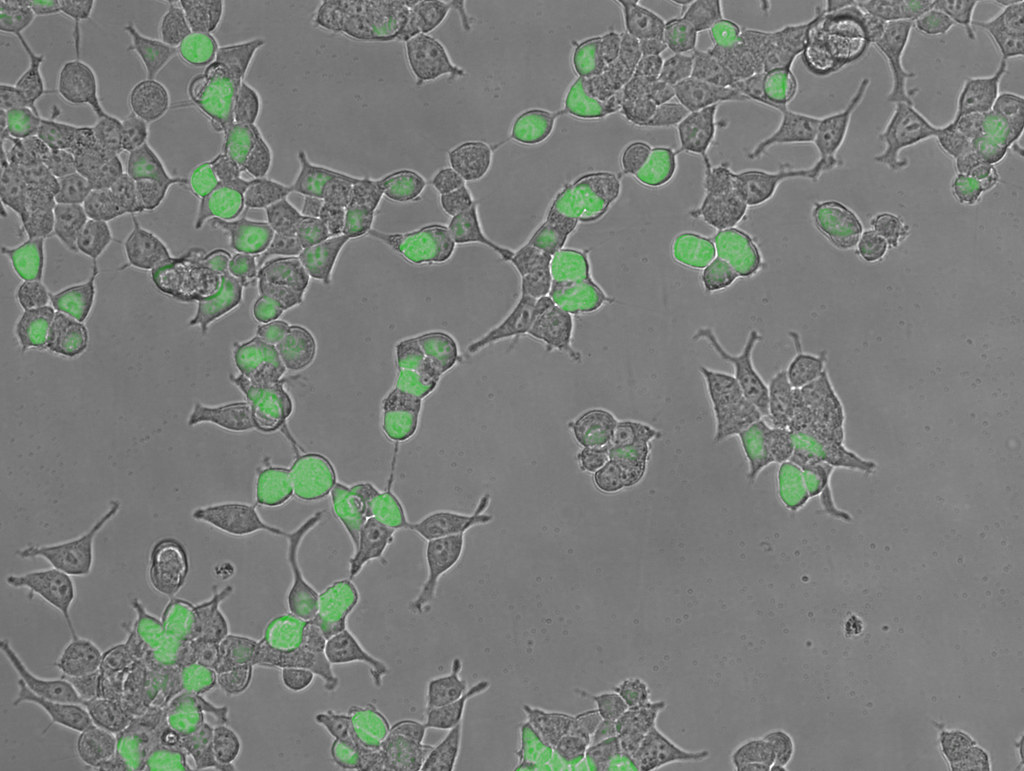
Higher magnification of HEK293T expressing the green fluorescent protein.
Plasmid Structure
pTarget mammalian expression vector. The MCS is inside the LacZ gene which permits for blue/white screening of the bacteria after cloning. The LacZ runs in the opposite orientation as the CMV promoter which will drive the gene transcription when inside a mammalian cell. SV40 origin servers as a plasmid replication origin if the cell line also expresses SV40 large T antigen, such as HEK293T cells.
Mammalian expression vectors contain the same hallmark features as bacterial plasmids: bacterial replication of origin and bacterial antibiotic resistance gene (β-lactamase or AmpR). General bacterial plasmid features allow for the carrying and the propagation of the plasmid in a bacterial cell. Mammalian expression vectors additionally include a strong mammalian promoter (like CMV from the cytomegalovirus immediate early promoter) upstream of a multiple cloning site (MCS). Plasmids transfected into cells are transient in nature unless the DNA is selected for. The inclusion of a mammalian antibiotic resistance gene, like neomycin phosphotransferase (NeoR), allows for the integration of the plasmid into the genome of the cell by using high concentrations of Neomycin or the analog G418.
Lipofection
Cationic lipids can encapsulate plasmid DNA in liposomes. The cationic portions interact with the negatively charged plasma membrane to deliver the DNA into cells.
Calcium Phosphate Transfection
Calcium chloride solution can be used to incubate with plasmid DNA. When this solution is mixed with a HEPES-buffered saline solution (HeBS) containing phosphate ions, the solution precipitates onto the surface of mammalian cells where they are taken up with the DNA.
Knock-Out & Transgenesis
In the laboratory, model organisms are modified in order to understand the basic mechanisms of genes. The transformation of recombinant DNA into bacteria is an example of a genetic modification. Other model organisms, like mice, are used to study genes. Through recombinant DNA scientists can selectively ablate a gene, or create a knock-out (KO).
Embryonic stem (ES) cells are pluripotent cells with the capacity to differentiate into other cell types. Cultured ES cells can be transfected with plasmid DNA in order to genetically alter them. Linearized vectors containing a disrupted gene can homologously recombine with the native gene to replace it. Selection of cells with the disrupted gene by an antibiotic (like G418) enables the isolation and propagation of engineered ES cells.
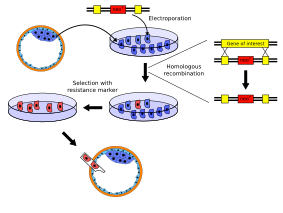
Credit: Kjaergaard (CC-BY- 3.0)

Credit: Kjaergaard (CC-BY- 3.0)
ES cells can be injected into mouse blastocysts and partially contribute to the subsequent mouse upon implantation into a mouse. These first mice are referred to as chimeras because they arise from mixtures of cells from 2 genetic sources. Germ-line transmission of the modified cells is desired and breeding of the chimera reveals heterozygous offspring of the engineered background. Full knock-out mice can be generated in the subsequent generation of breeding.


Credit: Smartse (CC-BY-SA 3.0)
Scientists can also overexpress or heterologously express foreign genes in what is termed transgenic organisms. As the name sounds, transgene refers to a gene from one place brought across into another.
Transgenic and KO models permit scientists to study the roles of genes inside the organism and understand basic functioning.
Through mutagenesis, derivatives of the green fluorescent protein (GFP) have been produced to provide a palette of colors. Additionally, the subsequent discovery of similar genes from other cnidarian species have aided biotechnology by providing tracer molecules within developing organisms or within cells.

Bacteria expressing various GFP derivatives on agar from the lab of Nobel Laureate Roger Tsien.
While commercial organisms like GloFish are a novelty, directed insertion of GFP and the variants into the genome under different promoter systems allow scientists to understand the cell-specific functioning or contribution to the organism. An example of this can be found in developmental neurobiology where individual axons can be traced.

A “brainbow” is a system where a cassette of GFP variant genes are placed downstream of a neuronal promoter to permit the tracing of individual neurons and their axons in mice. Credit: Jeff W. Lichtman and Joshua R. Sanes (CC-BY 3.0)
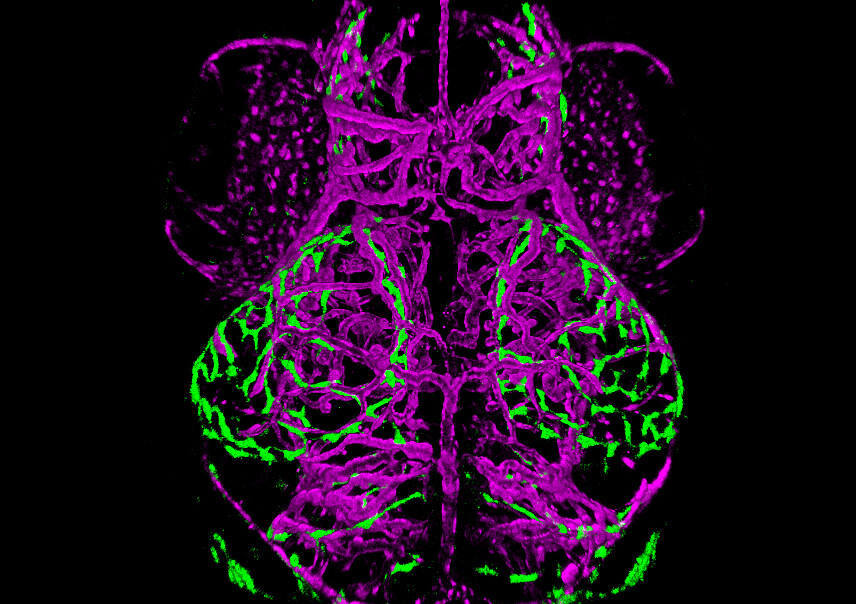
Brain of a 10-day old double-transgenic zebrafish. Blood vessels are shown in magenta (Kdrl:mcherry) and a novel population of perivascular endothelial cells are shown in green (MRC1a:eGFP).




