32.18: Turning Trees into Plexiglass - Synthetic Biology For Production of Green Foods and Products
- Page ID
- 110266
Search Fundamentals of Biochemistry
Introduction
Manufacturing of any kind is energy-consuming and environmentally damaging and contributes to climate change through the release of CO2 and other pollutants. A manufactured item has a lifetime after which it must be disposed of in a fashion that often involves little recycling. A circular economy in which a used product is always recycled for further use if done well, would be highly beneficial for the environment.
Synthetic biology as a field seeks to genetically alter and redesign organisms to produce traditional or novel products in more sustainable ways with less energy input and polluting output. Although it is a nascent field, well-known products are binding produced through its use. We will explore several products made through synthetic biology as well as several in which novel cells themselves are the products.
Products from cells
Burgers by Impossible Foods
Agriculture has literally transformed the planet. About 50% of all land (other than deserts and ice shields and comprising an area equal to the Americas) is used for agriculture and most of that is for animal production. In 2015, the US contributed about 8% of the world's total greenhouse gas emissions from food with China coming in at around 14%. Contributions to the production of food include agriculture, land use change, and supply chain emissions (transport, packaging, food processing, retail, cooking, and waste).
Estimates show that wild mammals comprise only about 6% of the total mass of all mammals (including people, livestock, and pets) on the planet). In a parallel finding, the mass of "stuff" (plastics, metals, asphalt, concrete, etc) created by humans now exceeds the entire biomass of the planet!
Most of the agricultural land is used to produce meat and milk for human consumption. Collectively, cattle by far require the most land use, as shown in the interactive graph of Our World In Data in Figure \(\PageIndex{1}\) below, which shows how many square meters are required to produce 1000 kcals (1000 cal in the dietary sense) of food from each food type listed. The graph is similar when the measure is land use per 100 grams of protein produced.
Figure \(\PageIndex{1}\): Land use of foods/1000 kcals. https://ourworldindata.org/land-use-diets
The number of animals slaughtered each day in the world is unbelievably high, as shown in Table \(\PageIndex{1}\) below.
| cows | goats | sheep | pigs | ducks | chickens | fish |
| 900,00 | 1.4 million | 1.7 million | 3.8 million | 11.8 million | 202 million | 100's of millions |
Table \(\PageIndex{1}\): Animals slaughtered each day for food. Max Roser (2023) - “How many animals get slaughtered every day?” Published online at OurWorldInData.org. Retrieved from: 'https://ourworldindata.org/how-many-...ered-every-day' [Online Resource]
Now if you consider what percentage of all GHG emission from a country come from the agricultural sector in that country, the numbers are closer to 20-30%, with the world average close to 35%, as illustrated in Figure \(\PageIndex{2}\) below.
Figure \(\PageIndex{2}\): https://ourworldindata.org/grapher/f...SA~IND~RUS~POL
The percent of greenhouse gas emissions from all aspects of food products through the entire food chain is about 25%. Figure \(\PageIndex{3}\) below shows the best estimate of global greenhouse gas per sector in 2016. The number today would probably differ little from these.
Figure \(\PageIndex{3}\): Breakdown of global greenhouse gas emissions in 2016. Hannah Ritchie (2020) - “Sector by sector: where do global greenhouse gas emissions come from?” Published online at OurWorldInData.org. Retrieved from: 'https://ourworldindata.org/ghg-emissions-by-sector' [Online Resource].
An expanded analysis of the approximate 25% of global emissions that arise from food is shown in Figure \(\PageIndex{4}\) below.
Figure \(\PageIndex{4}\): Global greenhouse gas emission from food production. Hannah Ritchie (2019) - “Food production is responsible for one-quarter of the world’s greenhouse gas emissions” Published online at OurWorldInData.org. Retrieved from: 'https://ourworldindata.org/food-ghg-emissions' [Online Resource]
Making plant-based foods that taste more like meat, if people would eat them, could have a large effect on greenhouse gas emissions and climate change. One example is the Impossible Burgers and other similar meats from Impossible Foods. They have soy leghemoglobin, a monomeric heme-binding protein found in root nodules in legumes, to give the appearance and taste of blood in meat. As a single-chain heme-binding protein, it has a high affinity for O2, similar to animal myoglobin. The high affinity derives from very high on-rates for binding O2 (almost diffusion-controlled at around 2x108 s-1, and an off rate of around 20 s-1. This high affinity keeps O2 bound which would otherwise inhibit nitrogenase and nitrogen fixation by root-associated microbes. The heme is important for positive tastes when we eat red meat. Plant-based burgers containing leghemoglobin require much less land and lead to far lower greenhouse gas emissions.
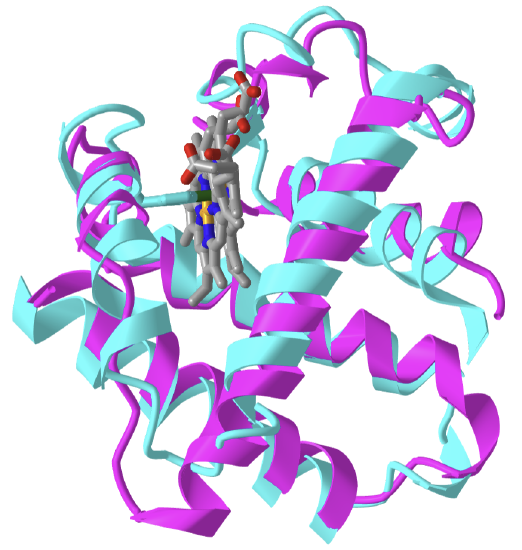
Figure \(\PageIndex{5}\) shows an interactive iCn3D model of the alignment of sperm whale myoglobin (1MBO) and soy leghemoglobin (1BIN).
Figure \(\PageIndex{5}\): Alignment of sperm whale myoglobin (1MBO, cyan) and soy leghemoglobin (1BIN, magenta). (Copyright; author via source). Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...4pUi1S6qFtGn4A
The leghemoglobin in the Impossible burgers is produced in yeast so it can be scaled up easily. To produce leghemoglobin in yeast, large amounts of heme are required, which is also produced in the engineered cells on the introduction of the appropriate genes. The heme synthesis pathways (described in Chapter 22.3) for C4 (humans, animals, fungi, and purple non-sulfur phototrophic bacteria top) and C5 (archaea, plants, and other bacteria) for heme synthesis are shown in Figure \(\PageIndex{6}\) below. The succinyl-CoA is derived from the citric acid cycle.
_pdftoPhotoshop.png?revision=1)
Figure \(\PageIndex{6}\): Top - heme synthesis pathway for C4 (humans, animals, fungi, and purple non-sulfur phototrophic bacteria top). Bottom - heme synthesis pathway for C5 (archaea, plants, and other bacteria). Heme biosynthetic pathway. Wikimedia Commonsile: Heme-Synthesis-Chemical-Details-Mirror (top) and Heme pathway in E. coli. Zhang, J., Kang, Z., Chen, J. et al. Optimization of the heme biosynthesis pathway for the production of 5-aminolevulinic acid in Escherichia coli. Sci Rep 5, 8584 (2015). https://doi.org/10.1038/srep08584. Creative Commons Attribution 4.0 International License. http://creativecommons.org/licenses/by/4.0/ (bottom).
Note that both succinyl-CoA (C4 pathway) and α-ketoglutarate (C5 pathway) are derived from the citric acid cycle. The key precursor 5-aminolevulinate, ALA needs to be elevated, through engineering of the C4 or C5 pathways with the C5 pathways generally producing more ALA in engineered E. Coli.
Leghemoglobin from soy (species name Glycine max) can also be synthesized in the methylotrophic (uses methanol as a sole carbon source) yeast Pichia pastoris which is often used for recombinant protein expression. Three groups of enzymes are needed"
- (group 1: porphobilinogen synthase (PBGS)
- group 2: uroporphyrinogen III synthase (UROS), uroporphyrinogen III decarboxylase (UROD), coproporphyrinogen III oxidase (CPO)
- group 3: Ala synthase (ALAS), protoporphyrinogen oxidase (PPO), and ferrochelatase (FECH)
Transcription of these genes in P. pastoris can be controlled by the use of the methanol-induced alcohol oxidase (AOX1) promoter, which is often used to achieve high expression of recombinant proteins. Hence when the cells also contain two copies of leghemoglobin along with the rest of the genes, high levels of the protein were made.
A more detailed representation of the heme synthesis pathway is shown in Figure \(\PageIndex{7}\) below.
Figure \(\PageIndex{7}\): The biosynthetic pathway of heme. Su, H.; Chen, X.; Chen, S.; Guo, M.; Liu, H. Applications of the Whole-Cell System in the Efficient Biosynthesis of Heme. Int. J. Mol. Sci. 2023, 24, 8384. https://doi.org/10.3390/ijms24098384. Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/)
The two biosynthetic pathways of 5-aminolevulinic acid (bolded to indicate its importance) are shown in green (C4 pathway) and red (C5 pathway). The three downstream synthetic pathways of heme are marked with blue (CPD), indigo (SHD) and purple (PPD). Solid lines indicate single reactions and dashed lines indicate more than two reactions. The names of genes encoding the individual enzymes are in italics and some reactions have alternative genes. The abbreviations of the corresponding enzymes are shown in the grey rectangle. See Table 1 for a list of names and abbreviations for heme synthesis enzymes
Figure \(\PageIndex{8}\) below complements this figure and shows the synthetic biology strategies to enhance heme production.
Figure \(\PageIndex{8}\): Synthetic biology strategies to enhance heme production. Green, orange, and red color blocks indicate genes that need to be up-regulated, down-regulated, and knocked out, respectively. Su, H. et al., ibid.
Computational tools such as AI can help the design of new pathways and novel enzymes to enhance production. It is becoming easier to transfer large pathways into yeast as well.
Other food products from microbes and sustainable plants
Significant effort is being devoted to growing meat in cell culture in the lab. This is a nascent field and has to overcome many problems, including consumer resistance to eating lab-created meat. At present meat grown in tissue culture is very expensive. Three key steps in growing meat are finding the best cells to grow, finding the nutrient conditions to maximize their growth, and adjusting conditions to make the lab meat taste like meat.
Muscle stem cells have been used as they can multiply many times, but these have growth limits. Alternatively, immortal cells, such as those derived from chicken fibroblasts, could be used. They can also be converted to fat cells. Yet they could accrue mutations with possible, but unlikely health consequences. Animal cells grown in culture often use fetal cow serum for its rich composition of growth factors and nutrients. However, it is very expensive and has ethical concerns as well since it's derived from animals. Synthetic growth medium can be used but it is also expensive. Whether lab-grown meat can overcome high costs and consumer resistance will determine its potential as a meat substitute.
More simply, people can use more peas, soy, grains, and nuts in their diet (i.e. being a vegan or vegetarian is the best approach to reducing your carbon footprint). Soy products have an extensive history of use as a source of protein but contain potential allergens (especially important in babies who use soy formulas) and isoflavones, which mimic human estrogen derivatives. Pea-based protein infant formulas are an increasingly used substitute.
Expressed recombinant proteins made in genetically modified bacteria and yeast are also becoming more popular. Examples (other than leghemoglobin) include the production in the fungus Trichoderma reesei of β-lactoglobulin, a cow whey protein, for dairy and animal-free milk products. The genetically modified yeast Pichia pastoris has also been engineered to make milk casein proteins, egg-white proteins, muscle myoglobin, and human breast milk proteins. Enzymes used in the manufacture of cheese (derived from calves' stomachs) can be replaced by chymosin made in yeast. Production is linked to fermentation for many of these proteins. Filamentous proteins that have a texture similar to chicken fiber can be made through fermentation in the filamentous fungi Fusarium venenatumin. Macroalgae like seaweed can provide high-protein food and have long been used in many cultures. Kelp farming can help not only provide protein but also capture carbon. Finally, insects, long eaten in many cultures, could become more climate-friendly sources of protein.
If humans are in search of nonanimal sources of protein to fight climate change, why not produce and eat the most abundant protein in the biosphere, Rubisco? New products derived from the duckweed plant (genus Lemna) are coming to market. Figure \(\PageIndex{9}\):
.jpg?revision=1&size=bestfit&width=506&height=380)
Figure \(\PageIndex{9}\): Duckweed (and a frog). https://commons.wikimedia.org/wiki/F...7678481%29.jpg
Duckweed is high in nutrients, fast-growing, and a great source of Rubisco. It can be grown in aquaculture and does not require farmable land. It contains up to 50% protein. After harvesting, the plants are filtered, milled, and dried, which are all very simple technologies. Proteins, the most abundant being Rubisco, are then extracted. It can be used in baked goods and as meat and dairy substitutes. It is equal to eggs and meat in supplying all the essential amino acids required by humans.
Genetic Manufacturing of Industrial Feedstocks
Let's look at one example in which synthetic biology and computational techniques are used to create products such as plexiglass from a biological source of acrylates. Acrylates are esters of acrylic acid (typically made from propylene) synthesized by reacting it with alcohols like methanol. Life cycle analyses show that almost 4000 kg of CO2 are produced per metric ton of acrylic acid made. To reduce the climate effect, biological feedstocks like glycerol and 3-hydroxypropanoic acid can be used, but large-scale supplies are needed. Figure \(\PageIndex{10}\) shows an overview of acrylate production fossil and biological feedstocks.
Figure \(\PageIndex{10}\): Production pathway of acrylates using fossil fuel and renewable resources. Souza, L.R.d.; Whitfield, B.; Al-Tabbaa, A. Biobased Acrylate Shells for Microcapsules Used in Self-Healing of Cementitious Materials. Sustainability 2022, 14, 13556. https://doi.org/10.3390/su142013556. Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/)
When the alcohol is methanol, the final product is methylacrylate (MA). The structure of the cyclic acrylate monomer feedstock used to polymerize plexiglass (lucite) is methylene-butyrolactone, (MBL)whose structure is shown in Figure \(\PageIndex{11}\) below.
Figure \(\PageIndex{11}\): Structure of methyl methacrylate and its lactone (a cyclic ester)
Methyl methacrylate can undergo a free radical polymerization in the presence of an initiator (In.), as shown below in Figure \(\PageIndex{12}\).
Figure \(\PageIndex{12}\): Mechanism of free radical polymerization of MMA
This reaction can form large polymers like plexiglass. The market for acrylic acid, the feedstock for plexiglass, is estimated to reach 12 million metric tons by 2030.
MBL, the lactone of MMA, is made in tulips from pathways that are not completely elucidated. It can also be used as a feedstock for the polymerization of plexiglass. Figure \(\PageIndex{13}\) shows the polymerization products from MMA and MBL.
Figure \(\PageIndex{13}\): Structure of poly-MMA and poly-MBL
Using synthetic biology and advanced computational methods, plexiglass can now be made from biological sources instead of fossil fuels. To accomplish this, Azerda has designed synthetic pathways from millions of potential metabolic pathways (using a software package called Scylax™), and intelligently redesigned key enzymes to maximize their catalytic potential for the synthesis of MBL (using the software Archytas™). They used high-throughput DNA and protein analyses to maximize expression. Finally, they engineered expression strains and downstream purification processes to maximize the final output of MBL. In summary, the key steps in the process were:
- identifying a pathway from millions of reactions in databases of pathways that could produce MBL from simple sugars through a fermentation process;
- engineering pathway enzymes to greatly increase catalytic efficiency and decrease inhibition;
- producing test quantities of the products in cell strains;
- scaling up production to levels needed for purification and reactions of the MBL
- purifying sufficient amounts of MBL from large fermentation broths
- making the desired product (plexiglass, for example) from the feedstock.
Strains of bacteria, yeast, and filamentous fungi were modified to meet the above criteria. The ultimate substrate for the process was a lignocellulosic hydrolysate, so in the end the process converts trees to plexiglass (incredible to think about)! Of course, it is also amazing that CO2 from the air, water, and minerals/ions from the soil can become a tree!
Starting with just a detectable level of product, the process was continually improved and scaled to eventually yield 5 g/L of broth, which is getting close to the 20 g/L required for commercial viability. Figure \(\PageIndex{14}\) below shows plexiglass created from the lignocellulosic stock!
Figure \(\PageIndex{14}\): Plexiglass made from biosourced MBL.
Table \(\PageIndex{2}\) below compares the key physical properties of the polymers from Arzeda's PMBL compared to literature values for fossil-fuel-based PMBL and for PMMA.
| Property | Measure | Lit PMBL | Arzeda PMBL | PMMA |
| Thermal | Glass transition pt Tg (oC) | 194-195 | 195 | 105 |
| Mechanical | Elasticity (mPa) | 1999/3439 | 5972 | 2855 |
| Tensile strength (mPa) | 36.7/62.7 | 72.7 | 70 | |
| Elongation at break | 1.3%.6.5% | 1.3% | 2.5 | |
| Optical | Light transmission | NA | >88% | 92% |
| Solvent resistance | toluene, 30 days, 20oC | NA | Pass | Fail |
Table \(\PageIndex{2}\): https://www.energy.gov/sites/default...-korkegian.pdf