32.8: Biodiesel, Syngas and Bioaviation fuels
- Page ID
- 102053
Search Fundamentals of Biochemistry
Diesel fuel was used in 1900 in an engine designed by Rudolf Diesel. The fuel was peanut oil. That might have worked fine in 1900, but a century later, the demands for diesel fuel are met not by peanut oil, a legume, but by oil. Gasoline (petrol) consists of molecules containing 5-12 carbons compared to diesel at 12-20. Both are obtained through fractional distillation of oil (petroleum). Diesel has a higher boiling and melting point and releases more energy per liter (36.9 vs. 33.7 MJ) than gasoline. Regular gasoline contains about 17% n-alkanes, 32% branched alkanes, 5% cycloalkanes, 2% alkenes (olefins), and 30% aromatics. High octane gas can contain around n- and branched alkanes, with the rest from alkenes. Diesel fuel contains about 75% saturated hydrocarbons and 25% aromatics, including alkylbenzenes and napthalenes. In a diesel engine, ignition occurs on compression of the fuel and air mixture and doesn't require a spark. They use glow plugs which provide heat but not spark.
Biodiesel
Oils (triacylglycerol) produced from biomass can be converted to diesel fuel. In the following sections, we will discuss the synthesis of gases and liquid fuels from nonpetroleum sources such as biomass through the creation of the synthetic gases H2 and CO (collectively called syngas) and their condensation into liquid fuels using the Fischer-Tropsch reaction. This section will limit our discussion to using fats to create biodiesel. Triacylglycerols for biodiesel production can come from plants, animals, algae, and even waste oils from the food industry. Because biodiesel fuel is composed of carbon-based molecules (often fatty acid esters) with high melting and boiling point ranges which hamper its utility in cold climates, it is often blended with regular diesel (for example, a 20% blend called B20). Still, it can be used at 100% (B100). Biodiesel enriched in unsaturated fatty acids has lower melting points and fewer problems in cold weather.
As will the production of bioethanol from lignocellulosic food stocks, biodiesel production has evolved through multiple generations, as shown in Figure \(\PageIndex{1}\) below.
Figure \(\PageIndex{1}\): Palani Vignesh et al., Oil Gas Sci. Technol. – Rev. IFP Energies nouvelles, 76 (2021) 6. DOI: https://doi.org/10.2516/ogst/2020088. Creative Commons Attribution License (https://creativecommons.org/licenses/by/4.0)
Table \(\PageIndex{1}\) below shows the advantages and disadvantages of each generation of biodiesel.
| Biodiesel generation | Advantages | Disadvantages |
|---|---|---|
| 1st generation biodiesel |
|
|
| 2nd generation biodiesel |
|
|
| 3rd generation biodiesel |
|
|
| 4th generation biodiesel |
|
|
Table \(\PageIndex{1}\): Advantages and disadvantages of various biodiesel generations. Palani Vignesh et al., ibid
Triacylglycerol feedstocks for biodiesel are often converted to methyl or ethyl esters through an alcoholysis or transesterification reaction, as shown in Figure \(\PageIndex{2}\) below.
Figure \(\PageIndex{2}\): Methanolysis/transestiferation of triacylglycerol to produce methyl-fatty acid ester for biofuels
This reaction is simply a base-catalyzed cleavage of the ester bonds in the triacylglycerol. Alternatively, vegetable oil can be treated at high pressure and temperature in a hydrogenation reaction to produce a variant called "renewable diesel".
Figure \(\PageIndex{3}\) below shows feedstocks and processing for first-generation biodiesel production.
| Feedstock: Processing Method | 1st Gen Processing |
|
Waste cooking oil: esterification/transesterification Food crops: extraction/transesterification Organic oils: hydrolysis, distillation Animal fat: hydrolysis, fermentation Bioethanol/butanol: chemical synthesis |
Figure \(\PageIndex{3}\): Feedstocks and processing for first-generation biodiesel production. Palani Vignesh et al., ibid
Figure \(\PageIndex{4}\) below shows feedstocks and processing for second-generation biodiesel production
| Feedstock: Processing Method | 2nd Gen Processing |
|
Cellulose: advanced fermentation Hemicellulose: hydrolysis Lignin: gasification Tannins: biological synthesis Vegetable oil/animal fats: hydrogenation |
Figure \(\PageIndex{4}\) below shows feedstocks and processing for second-generation biodiesel production. Palani Vignesh et al., ibid
Finally, Figure \(\PageIndex{5}\) below shows the processing steps for 3rd and 4th generation biodiesel from algae.
Figure \(\PageIndex{5}\): Processing steps for 3rd and 4th generation biodiesel from algae. Palani Vignesh et al., ibid
Let's consider 3rd and 4th generation biodiesel production using algae. First, algae can be used to produce many different products that can be used for biofuels and chemical feedstocks. These are reviewed in Figure \(\PageIndex{6}\) below.
Figure \(\PageIndex{6}\): Macroalgal biofuel refinery; Godvin Sharmila V et al., Bioengineered. 2021 Dec;12(2):9216-9238. doi: 10.1080/21655979.2021.1996019. PMID: 34709971; PMCID: PMC8809944. Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/)
To maximize biodiesel production from algae (3rd and 4th generation), steps in the anabolic pathways for fatty acid and triacylglycerol synthesis could be genetically modified. Below is an overview of triacylglycerol synthesis in microalgae in Figure \(\PageIndex{7}\).
Figure \(\PageIndex{7}\): Schematic illustration of TAG synthesis in microalgae. NADPH; Nicotinamide adenine dinucleotide phosphate; ATP, Adenosine Triphosphate; DHAP, dihydroxyacetone phosphate; G3P/G3pDH, Glyceraldehyde 3-phosphate / G3P dehydrogenase; GPAT, Glycerol 3-phosphate acyltransferase; PA/LPA/LPAAT/PAP, Phosphatidic acid/Lyso-PA/LPA acyltransferase/PA phosphatase; DAG/DGAT, di-Acylglycerol/ DAG acyltransferase; FAT, Fatty acyl-ACP thioesterase; ACP, Acyl-carrier protein; ER, Endoplasmic reticulum; PC, Phosphatidylcholine; PDAT, Phospholipid: DGAT; ACCase, Acetyl-CoA carboxylase; FAS, Fatty acid synthase; KAS, 3-ketoacyl-ACP synthase; FAD, Flavin adenine dinucleotide. Sharma PK, et al. Front. Mar. Sci. 5:382, 2018. doi: 10.3389/fmars.2018.00382. Creative Commons Attribution License (CC BY).
Each step in the combined pathways are sites for optimization, as shown in Figure \(\PageIndex{8}\) below.
Figure \(\PageIndex{8}\): Schematic illustration of different genetic engineering strategies applied in microalgae for biodiesel application. WT, Wild type cells; TR, Transgenic cells; TF, Transcription factor; TCA, Tricarboxylic acid cycle; NADH, Nicotinamide adenine dinucleotide; FA, Fatty acid; LD, Lipid droplet. Sharma PK, et al., ibid
Life cycle analyses for 3rd generation biodiesel production indicate they would lead to a net decrease in CO2 emissions, but most appear incomplete in their analyses.
Synthetic Gas (Syngas)
As an alternative to using fossil fuels as an energy stock to power our vehicles and as feedstock for chemical production, what if biomass could produce "gasoline-like" fuel for these purposes? We have already discussed the production of bioethanol from 1st (plant starch), 2nd (lignocellulose) and 3rd (algal) generation feedstocks. It is routinely added to gasoline to upward of 15%. It is also found in E85 (or flex fuel), a gasoline blend containing 50% to 80% ethanol.
Instead of producing ethanol through the fermentation of glucose, wood could be incompletely burned to create "synthetic gases" (CO and H2), called syngas, which could be further burned in vehicles to power them or converted through chemical processes (Fisher-Tropsh reaction) to liquid organic fuels.
Indeed, when fossil fuels were lacking, wood was used to create syngas power vehicles. Up to a million cars were powered by wood gas in Europe during World War II. A bus powered by wood gas (syngas) generated by a gasifier on a trailer is shown in Figure \(\PageIndex{9}\) below.

Figure \(\PageIndex{9}\): Bus power by wood gas c. 1943 in Leeds, England. By Ministry of Information Photo Division Photographer, Smith Norman? - http://media.iwm.org.uk/iwm/mediaLib.../large.jpgThis is photograph D 15675 from the collections of the Imperial War Museums., Public Domain, https://commons.wikimedia.org/w/inde...curid=24364067
The syngas emitted was cleaned up somewhat to remove tars and soot/ash particles by passing through charcoal before entering the vehicle through a tube. Tars with polycyclic aromatic hydrocarbons and methane could be lowered if wood or coals were first converted to char before use in a process called pyrolysis (heating to high temperatures in the relative absence of air).
The gases, derived from the incomplete combustion of the wood, have CO and H2 in various proportions depending on the temperature of burning and the source (wood, coal). A general and very simplified reaction for the incomplete combustion reaction is:
\begin{equation}
\text { Carbon feedstock }+\text { air } \rightarrow \mathrm{CO}+\mathrm{H}_2+\mathrm{CH}_4+\mathrm{CO}_2+\mathrm{H}_2 \mathrm{O}+\mathrm{N}_2
\end{equation}
Of course, the reaction is not clean, and many organic side products are produced.
The relative ratios of CO and H2 produced can be changed by the addition of water in a second reaction called the water gas shift (WGS) reaction (as water shifts the ratio of CO to H2):
\begin{equation}
\mathrm{CO}+\mathrm{H}_2 \mathrm{O} \leftrightarrow \mathrm{CO}_2+\mathrm{H}_2 \quad(\Delta \mathrm{H}=-41.2 \mathrm{~kJ} / \mathrm{mol} .)
\end{equation}
If run in reverse (rWGS) and at high temperatures, the water shift reaction would be a way to capture carbon. The H2 could come from the electrolysis of water
\begin{equation}
2 \mathrm{H}_2 \mathrm{O}(\mathrm{I}) \rightarrow 2 \mathrm{H}_2(\mathrm{~g})+\mathrm{O}_2(\mathrm{~g}) \quad(\Delta \mathrm{H}=286 \mathrm{~kJ} / \mathrm{mol})
\end{equation}
The electrocatalytic reduction of CO2 and H2O to produce syngas is shown in \(\PageIndex{10}\).
Figure \(\PageIndex{10}\): Syngas Generation by electrocatalytic reduction of CO2 and H2O. (after Kang Cheng et al. Advances in Catalysis, 60 (2017). https://doi.org/10.1016/bs.acat.2017.09.003
The oxidation numbers of each element are shown in the diagram. The cathode acts as a catalyst for the reaction. The reaction requires a power source, so this process is greener if electricity derived from green energy sources (wind/solar) is used.
Electrocatalytic CO2 reduction (ECR) offers the potential to capture CO2 before it is emitted into the air and convert it to small alkanes, alcohols, and acids for fuels (for example, methanol and ethanol) and chemical synthesis (for example, CO and formate). Again this would require a clean source of electricity to power these endergonic reactions. Table \(\PageIndex{2}\) below shows the standard reduction potentials for a variety of half-reactions that could be coupled to form the main ECR products.
Table \(\PageIndex{2}\): Lei Fan et al. Science Advances. 21 Feb 2020, Vol 6, DOI: 10.1126/sciadv.aay3111. Creative Commons Attribution NonCommercial License 4.0 (CC BY-NC).
The reactions are complex and require CO2 to adsorb onto the electrocatalytic surface of the cathode. Here is a possible reaction pathway for the conversion of CO2 to methane:
\begin{equation}
\mathrm{CO}_2 \rightarrow * \mathrm{COOH} \rightarrow * \mathrm{CO} \rightarrow * \mathrm{CHO} \rightarrow * \mathrm{CH}_2 \mathrm{O} \rightarrow * \mathrm{CH}_3 \mathrm{O} \rightarrow \mathrm{CH}_4+* \mathrm{O} \rightarrow \mathrm{CH}_4+* \mathrm{OH} \rightarrow \mathrm{CH}_4+\mathrm{H}_2 \mathrm{O}
\end{equation}
The catalysts employed are heterogeneous (i.e., not solution phase) and are typically organometallic transition metal structures. Possible reaction pathways to produce small CO2 electrochemical reduction products are shown in Figure \(\PageIndex{11}\) below.
Figure \(\PageIndex{11}\): Possible reaction pathways to produce small CO2 electrochemical reduction products. Lei Fan et al., ibid
This technology is early in development and will require the development of more robust catalysts and cells before it becomes commercially viable.
The synthesis of syngas (typically described as a mixture of CO, CO2, and H2) is widespread now, is used in various processes, and is used to make many products, including hydrocarbons for fuel and oxygen-containing derivatives, including methanol and ethanol. CO and H2 react in the Fischer-Tropsch reaction (described below) to produce alkanes and alkenes.
\begin{equation}
\mathrm{CO}+2 \mathrm{H}_2 \rightarrow\left(\mathrm{CH}_2\right)+\mathrm{H}_2 \mathrm{O} \quad \Delta \mathrm{H}=-165 \mathrm{~kJ} / \mathrm{mol}
\end{equation}
where CH2 is a methylene repeat in longer-chain alkanes.
The CO2 produced in syngas can be somewhat selectively removed by adsorption onto a CaO catalyst, as shown in Figure \(\PageIndex{12}\) below.
Figure \(\PageIndex{12}\): Adsorption configurations of CO2 on the surfaces of CaO-based catalysts at 650 °C: (a) CO2 adsorption on CaO (100) surface; (b) CO2 adsorption on 10 wt % Ni/CaO (100) surface. Green, red, gray, and purple balls represent Ca, O, C, and Ni atoms. Zhao, B. et al. Catalysts 2019, 9, 757. https://doi.org/10.3390/catal9090757. Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/)
The adsorption energies of CO, CH4, and H2 are so small that they have little effect on CO2 adsorption.
At present, the easiest way to make syngas is to react natural gas (methane) and steam under very high temperatures (up to 1000oC) over a Ni catalyst (a process called steam reforming). This results in a high H2/CO ratio of about 3. It can be done with liquified natural gas using Ni-ZrO2-CeO2-La2O3 catalyst. This process inherently would do little to decrease CO2 emissions. A gasification method converts coal, lignocellulosic biomass, and waste to syngas, with an H2/CO ratio of <1 for coal and about 0.6-1 for biomass. The electrocatalytic method described above has an H2/CO ratio of 0-2, depending on the nature of the cathodic catalyst. Syngas can also be made by the partial oxidation of methane. For the subsequent reactions (Fisher-Tropsch), the optimal H2/CO is about 2. In the gasification of lignocellulosic biomass, the water gas shift (WGS) is used to increase the H2/CO ratio. This requires lots of water and also produces CO2 as a product. The water shift reaction use catalysts such as Co–Mo–Al2O3, Fe2O3–Cr2O3, and Cu–ZnO–Al2O3) for coal gasification.
Syngas can be used to make small molecule energy and chemical feedstocks, such as ethanol (as well as liquid alkanes and alkenes). Given the many different types of products, it is often essential to selectively make and purify products for commercial use. One method for ethanol production is shown in Figure \(\PageIndex{13}\) below.
Figure \(\PageIndex{13}\): Conversion of syngas to ethanol proceeds through a tandem mechanism via methanol and acetic acid intermediates using a variety of sequentially positioned catalysts. Kang, J., He, S., Zhou, W. et al. Single-pass transformation of syngas into ethanol with high selectivity by triple tandem catalysis. Nat Commun 11, 827 (2020). https://doi.org/10.1038/s41467-020-14672-8. Creative Commons Attribution 4.0 International License. http://creativecommons.org/licenses/by/4.0/.
H-MOR is a zeolite, which is a microporous, crystalline structure made of aluminosilicate.
In a steam reforming reaction, bioethanol could be converted to CO and H2 as well, as shown in the equation below.
\begin{equation}
\mathrm{C}_2 \mathrm{H}_5 \mathrm{OH}+\mathrm{H}_2 \mathrm{O}(+\text { heat }) \rightarrow 2 \mathrm{CO}+4 \mathrm{H}_2
\end{equation}
Even with the ability to capture CO2 from the synthesis of syngas, a central issue of concern is whether the production of syngas and syngas-derived fuels and their use is associated with lower net CO2 emissions. A life cycle analysis would be necessary to determine that.
Fischer-Tropsch Synthesis (FTS) of Fuels
Fischer was head of the Kaiser-Wilhelm Institute for Coal Research in Germany at the start of World War I. Germany had abundant coal but needed oil for the war, so his efforts were redirected toward that end. Fischer and Tropsch developed the water shift reaction discussed above. They deployed new cobalt catalysts to produce oil which ultimately covered 25% of car fuel and 10% of the German military fuel needs in World War II. Large amounts were also made in South African during the Apartheid regime as well.
The Fischer–Tropsch synthesis (FTS) is a polymerization-like reaction that is used to convert gas-to-liquids (GTL), coal-to-liquids (CTL), or biomass-to-liquids (BTL) fuels. It starts with syngas (H2 and CO) produced from the gasification of coal/biomass or steam reforming/partial oxidation of natural gas), with the ratios of H2/CO determined by the water-shift reaction. If coal or biomass is used, a cleanup of residual products with heteroatoms and metal ions is necessary. The clean syngas is then passed into a reactor containing the required catalyst to the FTS of fuels. These reaction systems are summarized in Figure \(\PageIndex{14}\).
Figure \(\PageIndex{14}\): A simplified diagram of the Coal-to-Liquids (CTL), Gas-to-Liquids (GTL), and Biomass-to-Liquids (BTL) processes. Shafer, W.D.; Gnanamani, M.K.; Graham, U.M.; Yang, J.; Masuku, C.M.; Jacobs, G.; Davis, B.H. Fischer–Tropsch: Product Selectivity–The Fingerprint of Synthetic Fuels. Catalysts 2019, 9, 259. https://doi.org/10.3390/catal9030259. Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
The FTS reaction is conducted at moderate temperatures and pressure to produce fuels (diesel and jet), lubricants, waxes, and chemical feedstocks. The main representative reactions are given by:
N-alkane production:
\begin{equation}
(2 \mathrm{n}+1) \mathrm{H}_2(\mathrm{~g})+\mathrm{nCO}(\mathrm{g}) \rightarrow \mathrm{C}_{\mathrm{n}} \mathrm{H}_{2 \mathrm{n}+2}+\mathrm{nH}_2 \mathrm{O}
\end{equation}
Alkene production:
\begin{equation}
(2 \mathrm{n}) \mathrm{H}_2(\mathrm{~g})+\mathrm{nCO}(\mathrm{g}) \rightarrow \mathrm{C}_{\mathrm{n}} \mathrm{H}_{2 \mathrm{n}}+\mathrm{nH}_2 \mathrm{O}
\end{equation}
The products can have from one carbon (CH4) to over 70, depending on the catalyst, T, P, and H2/CO ratio. The FTS is a polymerization reaction, which starts with adsorption of the gas to the catalytic surface, followed by multiple cycles of free radical initiation, propagation, termination, desorption, and reabsorption.
Since this book focuses on structure/function and reaction mechanisms, we would be remiss not to include at least a simplified mechanism for these complex reactions. Several mechanisms have been proposed. Since iron was/is used in the catalyst, and since iron can form iron carbides, Fisher proposed a carbide mechanism. A second enol mechanism has also been proposed. Both are shown in Figure \(\PageIndex{15}\) below.
Figure \(\PageIndex{15}\): Proposed mechanisms for the Fischer-Tropsch reaction. Left: A proposed FTS route based on the carbide mechanism; Right: A proposed FTS route based on the enol mechanism. M is the metal surface.
In the carbide mechanism, CO adsorbs on the metal catalyst and dissociates in C and O atoms that cover the surface. These are hydrogenated to form H2O and CH2 (methylene), and H2 adds as the reaction proceeds, as shown.
In the enol mechanism, CO adsorbs without dissociation into atoms. It reacts on the surface to surface-bound H atoms (that arise from the dissociation of adsorbed H2) to form hydroxymethylene (M-CHOH). This enol grows on condensation with adjacent hydroxymethylenes. The rate-determining step is the hydrogenation of adsorbed CO.
Another proposed mechanism, CO insertion, is shown in Figure \(\PageIndex{16}\)
Figure \(\PageIndex{16}\): A proposed FTS route based on the CO insertion mechanism.
CO in this model inserts into a bond from a hydrogen atom to a metal on the catalyst. The rate-limiting step here is the hydrogenation of CO to the CH2 methylene group. The assumed monomer for this mechanism is simply CO through its insertion into metal-carbon bonds.
Generally, the FTS reaction catalyst has either cobalt or iron ions. The metal catalyst can also be doped with potassium and copper ions and bind silica and alumina. Iron is abundant and cheap and better promotes the water-gas-shift reaction, so it is best for FTS synthesis of fuels from coal and biomass since syngas derived from them have a lower H2/CO ratio.
Chemical Synthesis of Food
If fuels can be synthesized from nonpetroleum sources and from agricultural waste, why not use the reactions described above (like syn gas production, the Fischer-Tropsch, and electrochemical reduction) to make food, not just fuel? The agricultural sector accounts for about 25% of total greenhouse gas emissions, so it is ripe (no pun intended) for novel ways to produce foodstuffs (think of them as feedstocks for human consumption).
It should be obvious from the chemistry, that the simplest type of food to create from these reactions is fats, and not the more synthetical and stereochemically complicated proteins and carbohydrates. Presently the world relies on palm oil derived from palm trees for fats found in crackers, cookies, breads. and other food products. Typical production of palm oil has been associated with large-scale deforestation of rainforests, with its associated climate and biosphere impacts. Americans consume about 8 kg (17.6 lbs) of palm oil products each year, including products like cosmetics, cleaning products, and waxes. Figure \(\PageIndex{17}\) below shows the scale of palm oil production in the world, which might come as a surprise since we more frequently encounter soybean, sunflower, rapeseed (source of canola oil), and olive oils in our stores.
Figure \(\PageIndex{17}\): Vegetable Oil Product in the World. Our World in Data. https://ourworldindata.org/palm-oil
The general chemical pathways used to chemically synthesize proteins, fat,s and carbohydrates are illustrated in Figure \(\PageIndex{18}\) below. The fat synthesis pathways relevant for this discussion are represented in the central part of the figure.
Figure \(\PageIndex{18}\): Schematic of potential pathways to synthesize food without agriculture. Davis, S.J., Alexander, K., Moreno-Cruz, J. et al. Food without agriculture. Nat Sustain (2023). https://doi.org/10.1038/s41893-023-01241-2. Creative Commons Attribution 4.0 International License. http://creativecommons.org/licenses/by/4.0/.
Proteins, fats, and carbohydrates can be synthesized from a range of carbon feedstocks via multiple chemical and biological pathways (arrows). The weight and color of the arrows indicate the scale at which the different processes have been demonstrated and the energy required per mass unit output, respectively. Dashed lines indicate where energy requirements remain highly uncertain. Circular labels on each arrow further indicate whether the process is typically continuous (C) or batched (B). NH3 is ammonia, H2 is hydrogen gas, MeOH is methanol, and AcO- is acetate (agriculturally produced carbon feedstocks are omitted).
It is estimated that the chemical of synthesis of fats could lead to the emission of <0.8 g CO2 equiv/kcal compared to >1.5 g CO2 equiv/kcal for the present biological synthesis and processing of palm oil in Brazil or Indonesia. This advantage would increase if the CO2 for the chemical synthesis could be captured from the atmosphere, yielding net 0 emissions.
Figure \(\PageIndex{19}\) shows both land use and energy emissions of CO2 equiv/kcal for agricultural-produced fats (panel a) versus equivalents for chemical-synthesized fats.
Figure \(\PageIndex{19}\): Comparison of emissions per calorie of edible fats. Davis, S.J et al., ibid.
Panels a and b show shading and contours show grams of CO2-equivalent GHG emissions per kilocalorie of edible fat produced by conventional agriculture (Panel a) and chemical synthesis (Panel b). Agricultural emissions are shown as the sum of land-use emissions (y-axis) and energy-related emissions (x-axis), and emissions from synthesis are shown as a function of feedstock emissions intensity (y axis) and energy emissions intensity. Red circles denote specific estimates based on literature and assumed values. Feedstock emissions include process-related conversion of feedstock to CO2—for example, during extraction of natural gas, gasification of coal, and the eventual human respiration of fossil feedstock.
Bioaviation Fuel
Now we can turn our attention to the production and analysis of bioaviation fuel, which is more like kerosene and diesel fuel than ethanol in its composition. As we saw for bioethanol productions, the feedstocks can be 1st, 2nd and 3rd generation, as shown in Table \(\PageIndex{3}\) below.
| First-generation (1-G) | Second-generation (2-G) | Third-generation (3-G) | Fourth-generation (4-G) |
|
|
|
|
Table \(\PageIndex{3}\): Feedstocks for bio-aviation fuel production. Doliente SS, Narayan A, Tapia JFD, Samsatli NJ, Zhao Y and Samsatli S (2020) Bio-aviation Fuel: A Comprehensive Review and Analysis of the Supply Chain Components. Front. Energy Res. 8:110. doi: 10.3389/fenrg.2020.00110. Creative Commons Attribution License (CC BY).
A typical jet aviation fuel (Jet A-1) contains n- and branched-alkanes (often called paraffins) and some alkenes (often called olefins) with 8-16 carbon atoms, cycloalkanes, and aromatics. A comparison of the components of Jet-A1 with a typical bioaviation fuel, Bio-Jet, is shown in Figure \(\PageIndex{20}\) below.
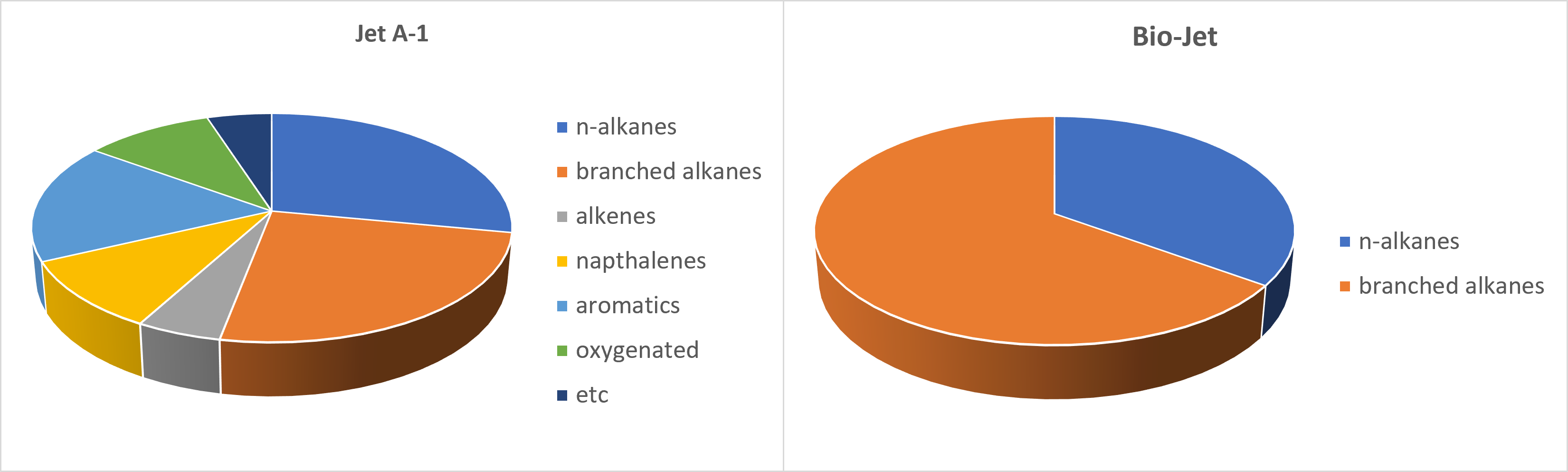
Figure \(\PageIndex{20}\): Molecular-class compositions of (a) Jet A-1 and (b) bio-jet identified by the relative signal area percentage analysis of GC–MS. After Cheon Hyeon Cho, Hee Sun Han, Chae Hoon Sohn, and Jeong Sik Han. ACS Omega 2021 6 (40), 26646-26658. DOI: 10.1021/acsomega.1c04002.
Bioaviation fuel can be made from feedstocks containing triacylglycerol or, most readily using feedstocks shown in Table 2 above to create syngas for use in the Fischer-Tropsch reaction. We've already discussed those reactions above. Instead, let's focus on whether bioaviation fuel, better termed sustainable aviation fuels (SAF), is good for our climate. This presupposes that battery-powered planes and jets are not scalable to our current environmental needs.
Life Cycle Analysis - Jet Fuel from Grasses
As expected, the US is the largest user of aviation fuel and causes 25% of aviation CO2 emissions, as much as all the greenhouse gases emitted through fuel use in Spain. It is estimated that the US will need around 30 billion gallons/per (BGY) of jet fuel in 2040. A biojet fuel industry based on cellulose as a feedstock could theoretically produce that amounts. If the industry costs are estimated at $123 billion, then the cost of the jet fuel would be $4.30/gal. About 60% of the costs would arise from the conversion of biomass to sustainable aviation fuel (SAF), which we described above. Those costs include the building of the biorefineries. Their cost would be distributed over their lifetimes of the plants. The fuel would be derived from syngas from the Fisher-Tropsch reaction, a reasonably mature technology. The costs would be much lower (closer to $1/gal) if the infrastructure costs were not included. These total costs are comparable to the price paid for regular jet fuel (around $2.2/gal in 2021). Consumer costs would not go up 2-fold since fuels are only part of the cost paid by passengers (15-25%)
Consumers' current price for fossil fuel is much less than its actual cost. The present price/gal does not include external costs associated with the use of fossil fuels. These include climate change effects on infrastructure, agriculture, industry, etc., and on human health (mostly from negative health consequences and diseases exacerbated by fossil pollution). We all ultimately pay for these hidden costs resulting from a failed market for pricing fossil fuels. On top of this, the fossil fuel industry has been massively subsidized for decades.
Different prices have been placed on carbon emissions from fossil fuels to resolve this error in the market. The carbon price is based on the number of tons of CO2 equivalent emitted ($US/ton CO2e). If a reasonable carbon price is added, biojet fuels made from lignocellulosic stocks through the Fisher-Tropsch reaction would be theoretically competitive with conventional jet fuels made from fossil fuels. The extra added cost for sustainable aviation fuel (SAF) compared to traditional aviation fuel at different prices placed on carbon placed on each are shown in Table \(\PageIndex{4}\) below, assuming an average cost of $2.20/gal for traditional aviation fuel.
| Price on carbon ($US/t CO2e) | SAF - Traditional Aviation Fuel ($/gal) |
| 0 | +$1.90/gal |
| $50 | +$0.60/gal |
| $175 | $0 |
If the cost of traditional jet fuel went to $3/gal, as it did in the US in March 2022, SAF and conventional jet fuel would cost the same if a price on carbon of $100/t CO2e were included for both. The actual "social" cost of carbon has been calculated (9/22) to be $175/ton CO2e.
Other factors other than the cost of carbon should be included in these analyzes. These include the issue of sustainable land use to grow feedstocks for the SAF. A recent analysis shows that it would be possible to produce 30 billion gall/yr of cellulosic SAF by planting 23.2 Mha (Million hectares, about the size of the state of Wyoming) of marginal agricultural lands (about one-third of croplands and 2/3 noncrop lands) in the Midwest with miscanthus, with a net cost of $4.1/gal and assuming a carbon price of a $50/ t CO2e. Miscanthus is a rapidly growing tall perennial grass with a high yield that grows in moderate climates. The life cycle analysis included interactions among atmospheric, land surface, ecosystem, and economic systems. Miscanthus gigantheus is shown below in Figure \(\PageIndex{21}\).
Figure \(\PageIndex{21}\):Miscanthus gigantheus. https://commons.wikimedia.org/wiki/F...us_Bestand.JPG. Creative Commons Attribution-Share Alike 3.0 Unported
Figure \(\PageIndex{22}\) shows some data from the study. Four different scenarios are offered, each represented by bar graphs.
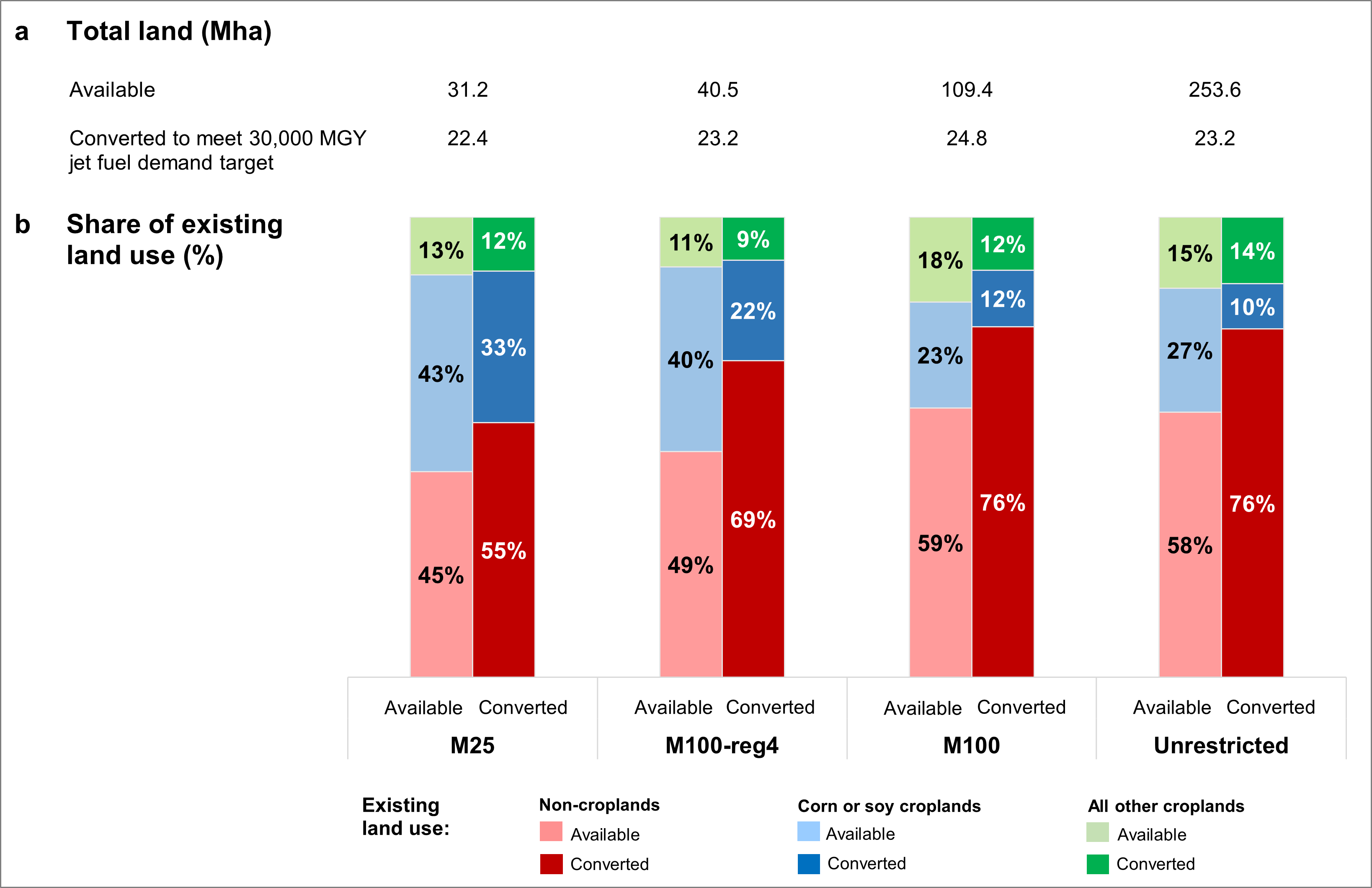
Figure \(\PageIndex{22}\): Land availability and conversion by existing use. Excel data and graph from https://dataverse.harvard.edu/datase...910/DVN/VBFLI2. CC0 1.0 Public Domain.
Four scenarios (left to right) were used to produce 30 MG/yr of SAF using a carbon price of $50/t CO2e.
- M25: only 25% of the marginally useful land was used
- M100-reg4: marginal land bases with the lowest hydrological and climatic risks
- M100: all of the marginally included land was made available
- Unrestricted
Panel (a) shows that in each case, about 23.2 Mha of land was converted to growing miscanthus out of the available land. Pane (b) shows stacked bars showing the percentages of each type of land available and converted for each scenario. The last scenario shows that demand can be made with the lowest % conversion of the marginal lands now used for corn/soybeans and other crops. It would appear that the marginal croplands converted to SAF production would be the same lands diverted to bioethanol production. Nevertheless, it would appear that up to 76% of projected aviation fuel needs could be met by planting marginal cropland and noncrop land for cellulosic SAF production. The study found that using available lands in the Plains was not feasible.
- Biodiesel, syngas and bioaviation fuel are alternative biofuels derived from biomass that can be used to reduce the dependence on fossil fuels.
- Biodiesel is a liquid fuel that can be used in diesel engines, it is produced by chemically converting vegetable oils or animal fats into a fuel that can be used in place of diesel fuel.
- Syngas (synthetic gas) is a mixture of hydrogen and carbon monoxide that can be produced from biomass through processes such as gasification. It can be used as a fuel for heat and power generation or further converted into chemicals and liquid fuels.
- Bioaviation fuel is a form of biofuel that can be used in aviation, it is made from biomass such as algae or woody biomass, it can be blended with traditional jet fuel and can help reduce emissions from airplanes.
- These biofuels have different advantages and limitations, and their production process is still in the research and development stage.
- Biodiesel has the advantage of being able to be used in existing diesel engines with little or no modification, it reduces emissions and it is renewable.
- Syngas has the advantage of being able to be converted into different chemicals and liquid fuels, it has a higher energy content than bioethanol and it can be used for power generation.
- Bioaviation fuel has the advantage of reducing emissions from airplanes, it can reduce dependence on fossil fuels in the aviation industry and it can be renewable.


