32.7: Algae - Bioethanol production
- Page ID
- 102048
Search Fundamentals of Biochemistry
- Understand the role that algae play in the production of bioethanol.
- Explore the process of converting algae into bioethanol.
- Learn about the potential benefits and drawbacks of using algae for bioethanol production.
- Evaluate the sustainability of algae-based bioethanol production.
- Analyze the potential of algae as a renewable source of bioethanol and its potential impact on climate change.
Now we are in a position to discuss algae as a source of biofuels and foods. Algae can be used to produce bioethanol from the fermentation of algal polysaccharides (starch, cellulose, and other unique polysaccharides found in them). In addition, triacylglycerols can be converted to biodiesel (which we will discuss in a different chapter). Since we explored bioethanol production from starch and cellulose in previous sections, we'll focus mainly on the unique carbohydrates found in algae and how they can be converted into monomers for glycolytic fermentation by yeast to ethanol.
Both microalgae and macroalgae contain starch reserves (in the cytoplasm and, in some cases, in the chloroplast) and cellulose (in cell walls). They can be grown quickly in aquatic environments, and the key molecules are harvested and processed for use in yeast fermentation by yeast and also by bacteria such as Zymomonas mobilis to produce bioethanol. This enzyme can use glucose, fructose, and sucrose as substrates.
Starch from microalgae
Green microalgae have been used as a source of both starch and cellulose. Some species of microalgae have very high starch/glucose composition by mass. Examples include Chlamydomonas reinhaedtil (60%), Chlorococcum humicola (33%), and Chlorella vulgaris (50%). Pretreatment of the microalgae biomass includes liquefaction using alpha-amylases followed by the addition of amyloglucosidase to produce glucose monomers (saccharification) or hydrolysis of glucosidic bonds using acid (sulfuric acid) or base (sodium hydroxide) pretreatment at elevated temperatures.
As discussed previously, starch consists of amylose, an unbranched glucose polymer with α(1,4) glucosidic links, and amylopectin, which contains α(1,6) branches. Algae starch (for example, in Chlorophyta, Cryptophyta, and Dinophyta) is found in the cytoplasm or chloroplast. A particular type of starch, Floridean (the primary energy storage molecule in the red algae Rhodophyceae), is also found in the cytoplasm. A generic structure of branched starches is shown below in Figure \(\PageIndex{1}\).
Figure \(\PageIndex{1}\): Branched starches (https://en.wikipedia.org/wiki/Floridean_starch.
Amylopectin has α(1,6) branches every 25-30 glucose units, while animal glycogen, another α(1,4) glucose polymer, has shorter branches every 8-12 glucose units. Floridean starch has branch lengths between these repeat values but is closer to amylopectin. β-amylases can cleave Floridean starch to mainly form glucose and maltose, while mild acid hydrolysis can lead to isomaltose formation.
Other unique glycans are potential sources of glucose for bioethanol production. These are described below.
Starch-like molecules in microalgae
Given algae diversity, it should be no surprise that some have alternative, starch-like glycans for their energy reserves that could also be used for bioethanol production.
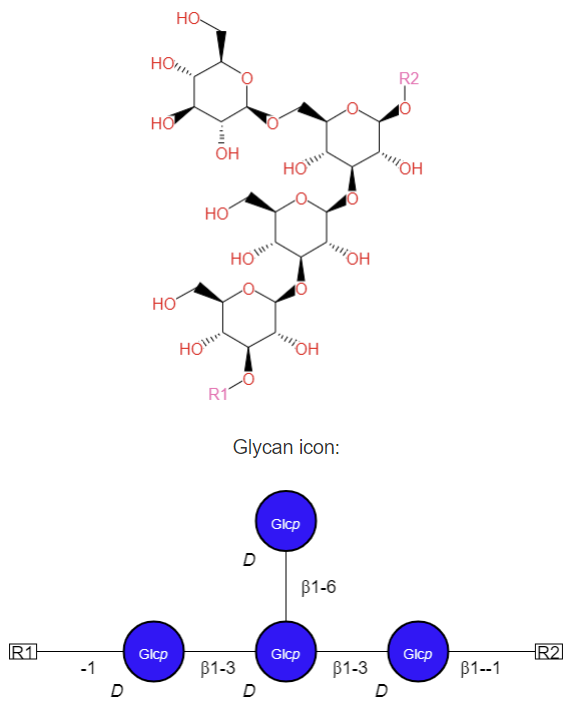
Laminarin (or Laminaran):
Laminaran is a linear polymer of glucose with β(1,3) glycosidic links with β(1,6)-branches at a ratio of 3:1. It is used for energy storage in brown algae. It can be hydrolyzed by laminarinase, which cleaves β(1,3) glucosidic bonds. It is a linear polysaccharide with a β(1→3):β(1→6) ratio of 3:1. Its structure is shown in Figure \(\PageIndex{2}\) below.
.png?revision=1&size=bestfit&width=465&height=570) |
Figure \(\PageIndex{2}\): Structure of laminarin. Left - https://commons.wikimedia.org/wiki/F...ructure_V1.svg; Right - https://biocyc.org/compound?orgid=ME...3602#tab=STRUC
Chrysolaminarin
This glycan is a linear polymer of glucose monomers linked through β(1,3) glycosidic bonds with some β(1,6) linkages. It is found in Haptophyceae, Bacillariophyceae, and Chrysophyceae, which include diatoms. Its generic structure is shown in Figure \(\PageIndex{3}\) below.
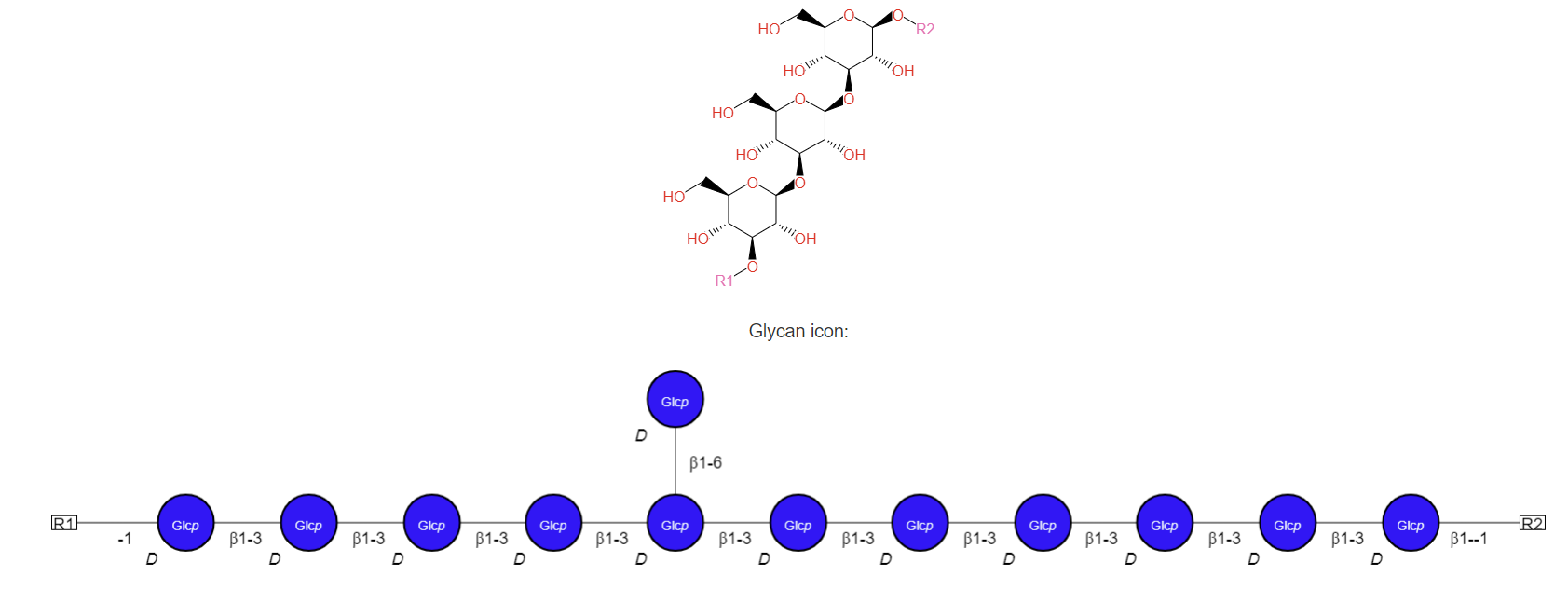
Figure \(\PageIndex{3}\): Structure of Chrysolaminarin. https://metacyc.org/compound?orgid=M...arin#tab=STRUC
The ratio of branching is 11/1, as indicated in the figure. Algae contain enzymes that can cleave the β(1,3) and β(1,4) links, which can be cleaved by acid hydrolysis.
Paramylon
Paramylon is a linear polymer of glucose monomers linked through β(1,3) glycosidic bonds. It is found in Euglenophyceae, Xanthophyceae, and Prymnesiophyta. A β-1,3 glucanase from Euglena gracilis (Euglenozoa) cleaves the β(1,3) link. Its structure is shown in Figure \(\PageIndex{4}\) below.
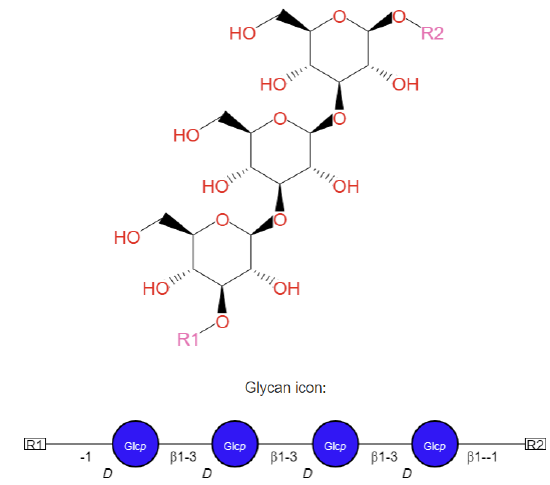
Figure \(\PageIndex{4}\): Paramylon. https://biocyc.org/compound?orgid=ME...ylon#tab=STRUC
Algae Cell Walls
In a previous chapter, we discussed the use of lignocellulosic feedstocks from plant cell walls to produce bioethanol. Both microalgae and macroalgae (red, green, and brown) have cellulose, a β(1,4) glucose polymer, in their cell walls. It is found in Chlorophyta, Dinophyta, Phaeophyta, Prymnesiophyceae, Rhodophyceae, and Xanthophyceae. There is little or much less lignin in algae and apparently none in macroalgae. In addition, there is less hemicellulose. These characteristics make algae excellent candidates for feedstocks for third-generation bioethanol production. There are problems to overcome as well. For example, the cell wall of Glaucocystis nostochinearum is almost 90% crystalline microfibrils, which adds to its physical and chemical stability. In addition, there are other glycans in some algae's extracellular matrix/cell wall, which can make it difficult to extract and use cellulose. Extracted cellulose can also be used as a feedstock for the chemical industry as well for the synthesis of bioplastics, a topic we will consider in another chapter sections.
Algae cellulose synthesis is performed by membrane-bound cellulose synthase terminal complexes (TCs), with the geometry of the cellulose microfibrils determined by the geometry of the TCs, as shown in Figure \(\PageIndex{5}\). The TCs are arranged in a hexagon with C6 symmetry, and they can form hexameric macrofibrils.
Figure \(\PageIndex{5}\): Organization and morphology of cellulose synthesizing terminal complexes (TCs) in different organisms. Wahlström, N. et al. Cellulose 27, 3707–3725 (2020). https://doi.org/10.1007/s10570-020-03029-5. Creative Commons Attribution 4.0 International License. http://creativecommons.org/licenses/by/4.0/.
Table \(\PageIndex{1}\) below shows the different algae taxa's major polymers in the cell wall and extracellular matrix.
| Algal taxa | Crystalline polysaccharides | Hemicelluloses | Matrix polysaccharides |
|---|---|---|---|
| Chlorophyta (green algae) | Cellulose | Xyloglucans, xylans, mannans, glucuronan, (1 → 3)-β-glucan, (1 → 3),(1 → 4)-β-glucan | Ulvans, pectins |
| Charophyceae (green algae) | Cellulose | Xyloglucans, xylans, mannans, (1 → 3)-β-glucan, (1 → 3),(1 → 4)-β-glucan | Pectins |
| Phaeophyceae (brown algae) | Cellulose | Sulfated xylofucoglucan, sulfated xylofucoglucuronan, (1 → 3)-β-glucan | Alginates, fucoidans |
| Rhodophyta (red algae) | Cellulose, (1 → 4)-β-mannan, (1 → 4)-β-xylan, (1 → 3)-β-xylan |
Xylans, mannans, glucomannans, sulfated (1 → 3),(1 → 4)-β-glucan, (1 → 3),(1 → 4)-β-xylan | Agars, carrageenans, porphyran |
| Dinophyta | Cellulose | – | – |
Table \(\PageIndex{1}\): Major polymers found in the cell wall and extracellular matrix of different algae taxa. Enio Zanchetta et al., Algal Research, 56 (2021). https://doi.org/10.1016/j.algal.2021.102288.
The percent composition of cellulose, hemicellulose and lignin for bioethanol production is important. Table \(\PageIndex{2}\) shows the % composition of these polymers based on total dry weight. (Note: The values for Chlorophyta/Ulvophyceae are highlighted in yellow in both Tables 2 and 3 for convenience of comparision.
| Empty Cell | Phylum/class | Strain | Cellulose [%] | Hemicellulose [%] | Lignin [%] |
|---|---|---|---|---|---|
| Microalgae | – | A mix of microalgae & cyanobacteria from the wastewater treatment plant | 7.1 | 16.3 | 1.5 |
| Chlorophyta/Trebouxiophyceae | Chlorella vulgaris | 10–47.5 | n.d. | n.d. | |
| Ochrophyta/Eustigmatophyceae | Nannochloropsis gaditana | 25 | det. | n.d. | |
| Macroalgae | Chlorophyta/Ulvophyceae | Cladophora glomerata | 21.6 | det. | n.d. |
| Chlorophyta/Ulvophyceae | Ulva lactuca | 12.4 6.0 16.6 |
n.d. 12.2 32.5 |
n.d. 9.8 1.5 |
|
| Chlorophyta/Ulvophyceae | Ulva prolifera | 19.4 | 14.4 | 9.4 | |
| Chlorophyta/Ulvophyceae | Ulva pertusa | 6.7 | 16.8 | n.d. | |
| Chlorophyta/Ulvophyceae | Ulva sp. | 40.7 | 7.1 | 7.9 | |
| Ochrophyta/Phaeophyceae | Cystosphaera jacquinottii | 4.6 | 6.1 | 19 | |
| Ochrophyta/Phaeophyceae | Fucus vesiculosus, Laminaria digitata | 8 | n.d. | n.d. | |
| Rhodophyta/Florideophyceae | Gelidium elegans | 17.2 | 29.5 | 4.5 | |
| Tracheophyta/Monocots | Posidonia oceanica | 32.5 31.4–40.0 40.0 |
23.3 21.8–25.7 20.8 |
28.2 29.3–29.8 29.8 |
|
| Tracheophyta/Monocots | Posidonia australis | 20.2 | 11.7 | 14.5 |
Table \(\PageIndex{2}\): Cellulose, hemicellulose, and lignin content in total dry weight basis of algal feedstock. n.d.: not determined, det.: detected (either directly or indirectly). Zanchetta et al, ibid.
Table \(\PageIndex{3}\) below shows the % composition in the cell wall (instead of total biomass) for each of the three polymers.
| Empty Cell | Phylum/class | Strain | Cellulose [%] | Hemicellulose [%] | Lignin [%] |
|---|---|---|---|---|---|
| Microalgae | Chlorophyta/Trebouxiophyceae | Chlorella pyrenoidosa | 15.4 | 31 | n.d. |
| Ochrophyta/Eustigmatophyceae | Nannochloropsis gaditana | 75 | det. | n.d. | |
| Charophyta/Zygnematophyceae | Staurastrum sp. | 72 | 4.0 | 1.2–5.6 | |
| Macroalgae | Chlorophyta/Ulvophyceae | Valonia ventricosa | 75 | det. | abs. |
| Chlorophyta/Ulvophyceae | Cladophora rupestris | 28.5 | abs. | n.d. | |
| Chlorophyta/Ulvophyceae | Ulva lactuca | 19 | det. | n.d. | |
| Chlorophyta/Ulvophyceae | Chaetomorpha melagonium | 41 | det. | n.d. | |
| Chlorophyta/Ulvophyceae | Enteromorpha sp. | 21 | det. | n.d. | |
| Ochrophyta/Phaeophyceae | Fucus serratus | 13.5 | det. | n.d. | |
| Ochrophyta/Phaeophyceae | Laminaria digitata | 20 | det. | n.d. | |
| Ochrophyta/Phaeophyceae | Laminaria saccharina | 18 | det. | n.d. | |
| Ochrophyta/Phaeophyceae | Halidrys siliquosa | 14 | det. | n.d. | |
| Ochrophyta/Phaeophyceae | Himanthalia lorea | 8 | det. | n.d. | |
| Rodophyta/Florideophyceae | Ptilota plumosa | 24 | det. | n.d. | |
| Rodophyta/Florideophyceae | Rhodymenia palmata | 7 | det. | n.d. |
Table \(\PageIndex{3}\): Cellulose, hemicellulose, and lignin content in the cell wall of algal feedstock. n.d.: not determined, det.: detected (either directly or indirectly), abs.: absent. Zanchetta et al, ibid.
Note the complete absence of lignin in the cell walls of macroalgae, but the previous table does show some presence in some macroalgae as a whole.
Other cell wall-associated glycans for bioethanol production.
Macroalgae offer an abundant source of other glycans, three of which we describe below. Each requires unique sets of enzymes to convert them to free glucose for fermentation and bioethanol production.
Alginates
These are among the most abundant biopolymers in the world and are the prime carbohydrate in brown seaweeds, where they can reach up to 40% of the dry mass. It is found in the cell walls of macroalgae. They are already used for food or other commercial uses. For example, they are used as thickening agents in food and are also used in the textile industries.
The alginate (high in brown algae) is a linear polymer of 1,4-β-D-mannuronic acid (M) and 1,4 α-L-guluronic acid (G) monomers, with stretches (blocks) of pure G, pure M, and mixed MG. Representative structures are shown in Figure \(\PageIndex{6}\) below.
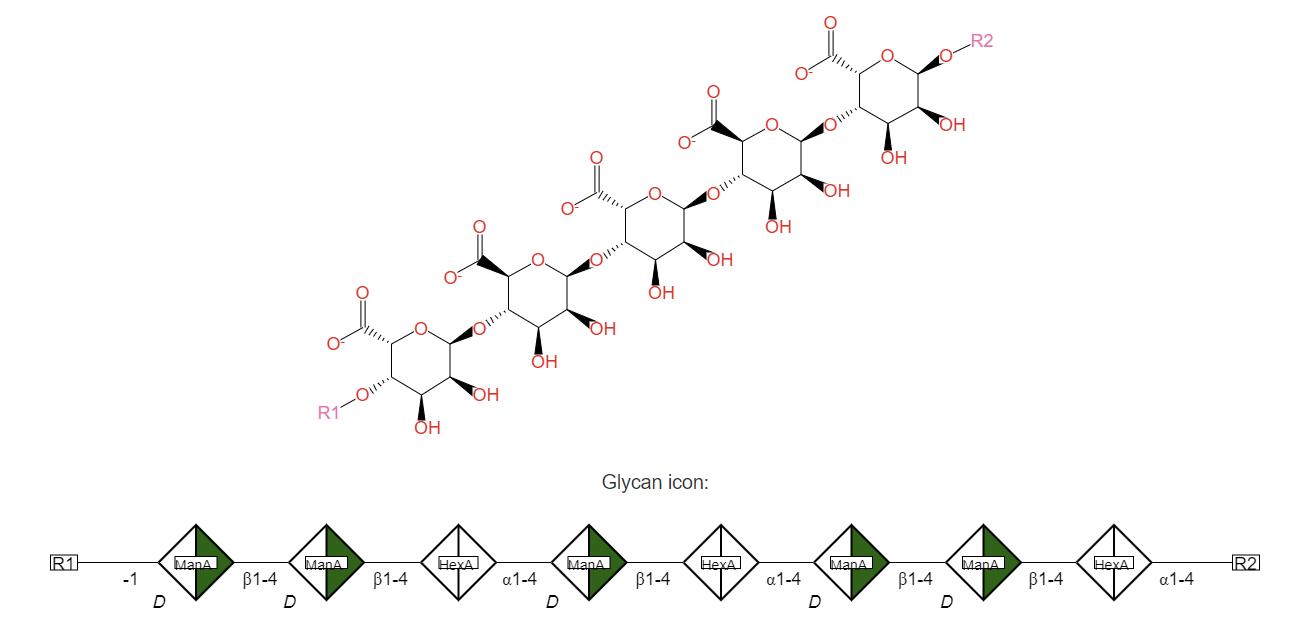 |
Figure \(\PageIndex{6}\): Left - https://commons.wikimedia.org/wiki/F...e_skeletal.svg; Right - https://biocyc.org/META/NEW-IMAGE?ty...bject=ALGINATE
Agar/Agarose
Agar is abundant in red seaweed. Agar, used in labs, is a mixture of agarose and agaropectin. It acts as a support for the cell wall and detaches with boiling. Agarose is a linear polymer of a disaccharide repeating unit of D-galactose and 3,6-anhydro-L-galactopyranose linked by α(1,3) and β(1->4) glycosidic bonds. It has many uses in the laboratory (agarose for chromatography and electrophoresis while agar for cell culture). Its structure is shown in Figure \(\PageIndex{7}\) below.
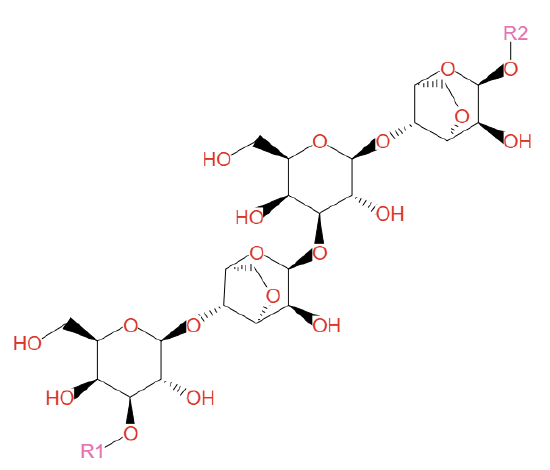 |
Figure \(\PageIndex{7}\): Agarose. Left - https://commons.wikimedia.org/wiki/F...e_polymere.svg; Right - https://biocyc.org/compound?orgid=ME...oses#tab=STRUC
Carrageenans
These are linear sulfated glycans found in the cell walls of red seaweeds (Rhodophyta ). They have widespread use in the food industry and are used for thickening. They are similar to glycosaminoglycans (GAGs). Their repeating motif is a disaccharide of an α(1,3)-linked D-galactose and a β(1,4)-linked D-galactose. As with GAGs, their degree of sulfation can vary from 15-40%. Different subtypes have a different number of sulfates in the repeating disaccharide unit. For example, the κ form has one, iota (ι) has two, and λ has 3. Representative structures are shown in Figure \(\PageIndex{8}\) below.
Figure \(\PageIndex{8}\): Carrageenans. https://upload.wikimedia.org/wikiped...enan_types.svg
Variants include the carrageenoses, in which the α(1,3)-linked galactose has a 3,6-anhydro bridge. The carrageenoses are often just called carrageenan. Their structure is shown in Figure \(\PageIndex{9}\).
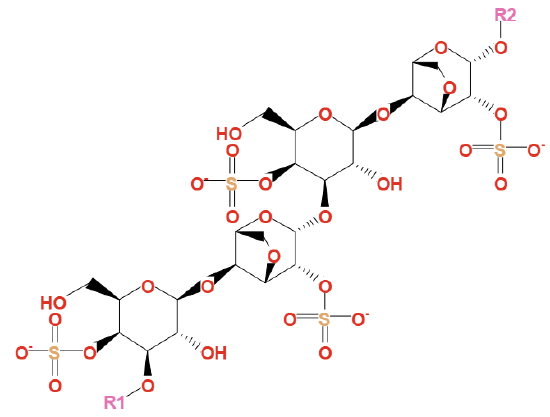
Figure \(\PageIndex{9}\): https://biocyc.org/compound?orgid=ME...enan#tab=STRUC
The 6-anhydro-D-galactose is not fermentable, so microorganisms that can contain enzymes that degrade carrageenan must also be used.
Bioethanol production
Now we can put all of this together and make bioethanol from algae. Figure \(\PageIndex{10}\) presents an overview of the entire process.
Figure \(\PageIndex{10}\): Overview of ethanol production from major algal carbohydrates. Qusai Al Abdallah et al., Front. Energy Res., 04 November 2016. https://doi.org/10.3389/fenrg.2016.00036. Creative Commons Attribution License (CC BY).
Panel (A) shows algae store simple sugars in the form of simple and complex food reserves and as structural polysaccharides.
Panel (B) shows how food reserves and structural polysaccharides are degraded into their basic monosaccharides and uronic acids.
Panel (C) shows the final fermentation into ethanol using microbial wild-type strains or their genetically engineered counterpartsDEHU, 4-deoxy-l-erythro-5-hexoseulose uronic acid.
Figure \(\PageIndex{11}\) below shows a schematic diagram for converting the algae feedstocks to glucose using key glycan-cleaving enzymes. Note that the colors and shapes are not those recommended in the SNFG standard. Those standards show glucose and galactose as blue and yellow circles, respectively.
Figure \(\PageIndex{11}\): SCHEMATIC DIAGRAMS FOR THE ENZYMATIC HYDROLYSIS OF ALGAL POLYSACCHARIDES. (A) Starch, Floridean starch, and glycogen, (B) laminarin, chrysolaminarin, and paramylon, (C) cellulose, (D) agarose by β-agarases, (E) agarose by α-agarases, and (F) alginate. DP, degree of polymerization; NAB, neoagarobiose; AB, agarobiose; DEHU, 4-deoxy-l-erythro-5-hexoseulose uronic acid; KDG, 2-keto-3-deoxy-gluconate; M, β-d-mannuronate; G, α-l-guluronaten.
Macroalgae offer enormous advantages for producing food, bioethanol, and intermediates for conversion into bioplastics. Ocean macroalgae don't require irrigation or pesticides, or fertilizers! Figure \(\PageIndex{12}\) below shows rope and raft ocean cultivation of macroalgae.
Figure \(\PageIndex{12}\): Macroalgae cultivation systems. Godvin Sharmila V et al. Bioengineered, Volume 12, 2021. https://doi.org/10.1080/21655979.2021.1996019. Creative Commons Attribution License (http://creativecommons.org/licenses/by/4.0/)
Figure \(\PageIndex{13}\) below summarizes how macroalgae (as well as microalgae) can be used to produce a variety of biofuels in addition to bioethanol. The algae cell can be chemically processed to produce hydrogen, methane, and syngas for fuels or combusted (as in the use of wood and coal) to provide heat and electricity. Alternatively, the post-harvest algae can be fractionated to produce carbohydrates for alcohol production, straight vegetable oils (SVO - pure triacylglycerols) for fuel, and fatty acid esters (biodiesel). Of course, they can be used directly as food and for food products.
Figure \(\PageIndex{13}\): Macroalgal biofuel refinery. Godvin Sharmila V et al., ibid.
| Type of Biofuel | Species Type | Pretreatment methods or Conversion techniques | Pretreatment or conversion technique conditions | Biofuel Yield or production potential |
|---|---|---|---|---|
| Biodiesel | Ulva fasciata | Catalytic transesterification | Molar ratio of methanol: oil – 9:1; Time – 6 hours; Temperature – 80-100°C | 88% |
| Chaetomorpha antennina | Transesterification | Chloroform-ethanol solvent- 1:20 (w/v) | 2.1 mL/10 gbiomass | |
| Gracilaria corticata | Transesterification | Hexane-ether solvent – 1:20 (w/v) | 2 mL/10 gbiomass | |
| Ulva intestinalis | Transesterification | - | 32.3 mg/g dw | |
| Enteromorpha compressa | Base transesterification | Base – 1% NaOH, Methanol–oil ratio – 9:1, Temperature – 60°CTime – 70 min | 90.6% | |
| Bioethanol | Chaetomorpha linum | Wet oxidation method | Temperature – 200°C | 44 g ethanol/100 g glucan |
| Saccharina japonica | Low acid pretreatment | Acid – 0.06% (w/w) sulfuric acidTemperature – 170°CTime – 15 min | 6.65 g/L | |
| Saccharinajaponica | Thermal acid hydrolysis | Acid – 40 mM H2SO4Temperature – 121°CTime – 60 min | 7.7 g/L | |
| Laminaria digitata | Oven drying | Temperature – 70°C,Time −72 h | 13.6 ± 0.2 μL/g DS | |
| Ulva linza | Mild acid hydrolysis | Acid condition – 3% H2SO4 | 12.01% | |
| Biohydrogen | Laminaria japonica | Microwave | Temperature – 160°C,Time – 30 min | 15.8 mL/g TS |
| Laminaria japonica | Ultrasonic | Sonication frequency – 20 kHz | 23.56 ± 4.5 mL/g | |
| Laminaria digitate | Hydrothermal | Temperature – 140°CTime – 20 min | 44.0 ± 1.2 mL/g VS | |
| Chaetomorpha antennina | Surfactant-aided microwave pretreatment | Microwave power – 0.36 kW Ammonium dodecyl sulfate – 0.0035 | 74.5 mL/g COD | |
| Ulva reticulate | Microwave-H2O2 alkali pretreatment | Microwave power – 0.36 kWH2O2 dosage – 24 mg H2O2/g biomasspH – 10 | 87.5 mL H2/g COD | |
| Biomethane | Palmaria palmata | Anaerobic digestion | Semi-continuous anaerobic digestion | 320 mL CH4/g VS |
| Chaetomorpha antennina | Ozone disperser pretreatment | Disperser g force – 1,613 g, Treatment time – 30 min, Ozone dosage – 0.00049 g O3/g TS | 0.20 g COD/g COD | |
| Chaetomorpha antennina | Thermo-chemo disperser | Disperser g-force of 1613 g, Temperature – 80°C, NaOH – 1 N, pH – 11 | 215 mL/g VS | |
| Laminaria digitata | Heat | Temperature – 104°C | Dried biomass – 97.66 m3 CH4/t fresh biomass – 67.24 m3 CH4/t | |
| Laminaria digitata | Oven drying | Temperature – 70°C,Time −72 h | 235.4 ± 14.1 mL/gVS | |
| Bio-oil | Saccharina japonica | Fixed bed reactor pyrolysis | Temperature – 450°C | 47% conversion |
| Ulva lactuca | Microwave pyrolysis | Temperature – 500°C | 18.4 wt.% | |
| Porphyra tenera | Packed tube reactor pyrolysis | Temperature – 500°C | 47.4 wt.% | |
| Laminaria japonica | Packed tube reactor pyrolysis | Temperature – 500°C | 45.8 wt.% | |
| Undaria pinnatifida | Packed tube reactor pyrolysis | Temperature – 500°C | 37.5 wt.% |
Table \(\PageIndex{4}\): Biofuel production from macroalgae. Godvin Sharmila V et al., ibid.
We will discuss in another chapter the production of biodiesel from algae.
- Algae can be used to produce bioethanol, a biofuel that is similar to traditional ethanol produced from corn or sugar cane.
- Algae-based bioethanol production involves the cultivation of algae, followed by the extraction of sugars and the fermentation of these sugars to produce ethanol.
- Algae have a high growth rate and high sugar content, making them a potential source of bioethanol.
- The process of bioethanol production from algae is being commercialized, and research is ongoing to improve the efficiency and cost-effectiveness of the process.
- Algae-based bioethanol production has several advantages over traditional bioethanol production methods, such as the ability to grow algae in non-arable land and the ability to produce bioethanol from CO2 and sunlight.
- The algae-based bioethanol production process, is considered more sustainable and environmentally friendly than traditional bioethanol production methods, as it does not compete with food crops for resources and consumes CO2 during production.


