Carbon Capture Using Carbonic Anhydrase
( \newcommand{\kernel}{\mathrm{null}\,}\)
|
Global Challenges
|
Climate Change Carbon Capture Using Carbonic Anhydrase |
Literature-Based Guided Assessment (LGA) |
Key Words, Concepts: protein structure, structure/function relationships, enzyme kinetics, enzyme mechanisms, reaction mechanisms, Western blot analysis, site-directed mutagenesis, biomolecular visualization, computational modeling, graphic analysis
For faculty: email for answers - hjakubowski@csbsju.edu
The Problem
Our climate is changing as planetary temperatures rise from increasing amounts of the greenhouse gas carbon dioxide released into the atmosphere (detailed in Chapter 31) on the burning of fossil fuels. The rate of release is unparalleled in geological history. Present levels (415 ppm) have not been seen for at least 3 million years. Human societies and cultures have had the opportunity to develop in relatively stable climatic conditions. Figure 1 below shows the rise in atmospheric CO2 over the last 1000 years.
Figure 1: CO2 levels in the atmosphere over the last 1000 years. Our world in data. https://ourworldindata.org/
The steep rise around 1790 coincides with the start of the Industrial Revolution. The rise in atmospheric CO2 has led to a corresponding rise in the average global temperatures, as illustrated in Figure 2 below.
Figure 2: Average global temperature changes over the last 1000 years. Our world in data. https://ourworldindata.org/
The per capita emissions of CO2 across the world derive from the use of coal, oil, and gas, as illustrated in Figure 3 below.
Figure 3: Per capita emission of CO2 from fossil fuel type
If we want to decrease emissions, we also need to know in which economic sectors fossil fuels are used. The main sources of global energy-related CO2 emissions by sector are shown in Figure 4:
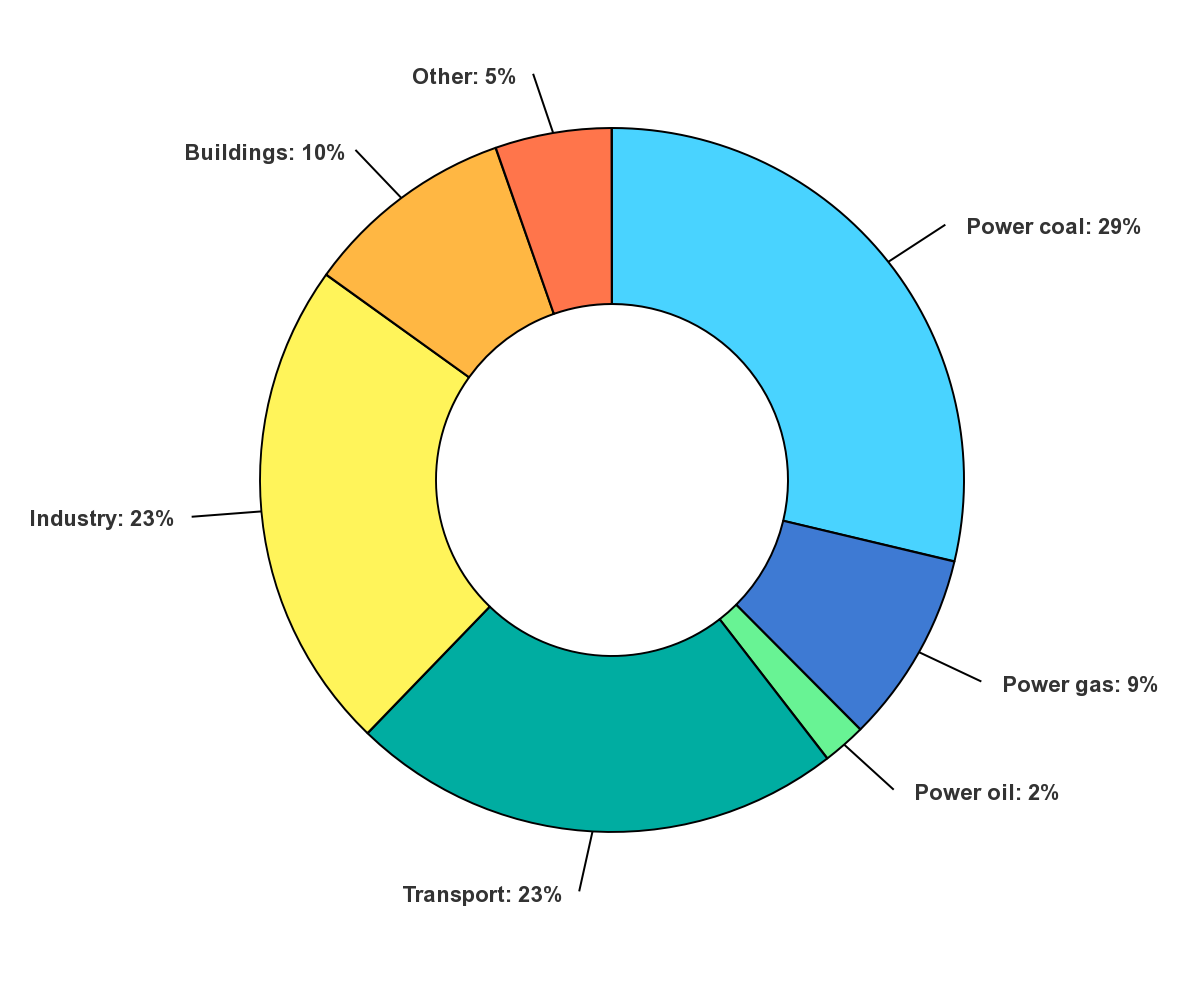
Figure 4: Global energy-related CO2 emissions by sector. Updated on 10/26/22. https://www.iea.org/data-and-statist...ions-by-sector. IEA. License: CC BY 4.0
Note the use of coal, gas, and oil for energy production (electricity) accounts for 40% of global CO2 emissions, with transportation (mostly through the use of gasoline and diesel fuel) and industry accounting for about 25% each.
Simple chemistry tells us that there are two ways to decrease the amount of product (in this case CO2) in a chemical reaction:
- decrease the concentration of reactants (i.e. reduce fossil fuel use)
- remove the product, in this case, CO2 from the air.
The latter process is called carbon capture or sequestration. It is a daunting process that nature has mastered (through photosynthesis), but it clearly can't keep up with the huge injection of CO2 in the atmosphere caused by burning fossil fuel.
We simply can't stop using fossil fuels, which would result in huge economic and social unrest. Alternative green fuels (solar, wind, for example) are being rapidly expanded but can't replace fossil fuels for many years. One of the reasons is that fossil fuels are very energy-dense (MJ/kg) compared to other sources of energy. It's also fascinating to look at the energy transitions humans have made over time. Figure 5 below shows the energy transition over a log-time scale (for presentation purposes) as well as the energy densities of individual sources.
Figure 5: Human-created energy transitions (log time scale) and energy densities of individual sources
New technologies are needed to capture CO2. We have to move much faster in a new clean energy transition than we have in our entire history. A potentially ideal solution would be to capture CO2 from power plants before they reach the atmosphere. We will now look at research into an old enzyme, carbonic anhydrase, that is being repurposed for industrial-level carbon capture, carbonic anhydrase.
Carbonic Anhydrase (CA)
We have already encountered this enzyme before (Chapter 6.1). It catalyzes the hydration of CO2 (g) as shown below.
CO2 (g) + H2O ↔ H2CO3 (aq) ↔ HCO3- (aq) + H+ (aq)
It is among the fastest of all enzymes, with a kcat of 106 s-1 and a kcat/Km of 8.3 x 107 M-1s-1 (reference). It is diffusion controlled in that the rate of diffusion of reactants and products, not the chemical steps, determine the reaction rate. It can convert 106 molecules of CO2(g) to HCO3- each second. No wonder scientists and engineers are studying it to capture CO2. It's a big challenge though to capture CO2 released on combustion of coal or natural gas in a power plant. Here are two problems that must be overcome:
- The enzyme must be thermostable at elevated temperatures to capture the CO2 found in high-temperature power plant emissions
- The enzyme is reversible so it will be inhibited by the product HCO3-
- The enzyme must be stable to somewhat alkaline conditions (pH of 0.1M NaHCO3 = 8.3)
For carbon capture from fossil fuel emissions, CA is immobilized by surface adsorption, covalent attachment, encapsulation, and entanglement. Immobilized enzymes are typically more thermostable and can be used in flow-through as opposed to solution phase capture. The immobilized enzyme matrix must withstand high temperatures (up to 100°C, and alkaline solvents used to strip the matrix for reuse.
The enzyme is found throughout life and typically has an active site Zn2+. There are 8 families, α, β, γ, δ, ζ η, θ, and ι, with the α family being the most abundant. The α forms are generally active as dimers, but can act as monomers and tetramers.. There are 15 isoforms of the α form in humans and have a prime role in pH regulation. They are found in bacteria, fungi, plants, and algae. β-CAs are found in some types of bacteria, archaea, fungi, some higher plants, and invertebrates. CA in chloroplasts (and mitochondria (algae) are involved in carbon fixation. We will focus our attention on engineering carbonic anhydrase to make them more thermostable, alkali insensitive, and less susceptible to product inhibition by bicarbonate.
Natural enzymes can be isolated and selected for thermal and alkali stability. In addition, new versions selected for these properties can be engineered using directed evolution or site-directed mutagenesis. You wish to increase the thermal stability of a protein using mutagenesis. Essentially you wish to perturb the equilibrium between the folded (native) protein and the unfolded (denatured) protein so as to preferentially stabilized the native state.
Using mutagenesis, what residues might you change in a native protein to make it more stable at higher temperatures?
- Answer
-
What measurements would you make to quantitate the change in thermal stability?
- Answer
-
The actual amino acid composition and more strangely specific dipeptide sequences within a sequence are associated with thermal stability of hyperthermophilic proteins. For example, proteins from two different types of archaea with different optimal growth temperatures show that the one with the higher growth temperature have significantly higher levels of VK, KI, YK, IK, KV, KY, and EV and decreased levels of DA, AD, TD, DD, DT, HD, DH, DR, and DG. Similar experiments have been done in bacterial cells. Using machine learning, the dipeptide sequences K∗H, KR, TF, P∗M, F∗∗N, V∗∗Y, MW, and WQ were important in themostability where the * denotes a gap in the residues.
Structure and Mechanism
An active site Zn2+ appears to bind a water molecule and reduce its pKa such that the bound form is OH-. This is illustrated in the left panel of Figure 6 below, which depicts the local environment of the bound Zn2+ (coordinated byhistidine side chains and an OH-) in the absence (left) and presence (right) of CO2. Note that the back histidine is difficult to barely visible (but still evident) in both structures. To assist in viewing the structure, the right panel shows an interactive iCn3D model of Zn- human carbonic anhydrase II at pH 7.8 and 0 atm CO (6LUW).
Figure 6: Left panel: Coordination of OH- to Zn2+ in carbonic anhydrase in the absence (left) and presence (right) of substrate CO2. Right panel: Zn- human carbonic anhydrase II at pH 7.8 and 0 atm CO (6LUW) (Copyright; author via source). Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...43DYyZFvHJpZn9
What is the coordination geometry of the Zn ion?
- Answer
-
Draw a simplified reaction mechanism showing bicarbonate formation from the two reactants, CO2 and OH-.
- Answer
-
The enzyme is reversible and in humans is important in CO2 transport in respiration and maintaining intracellular pH, which is also its key role in most organisms.
CO2, like the other atmospheric gases O2 and N2, are nonpolar and have limited solubility in water. The solubility of these gases in water at 20oC and 1 atm pressure, in g/L and mM, are shown in Table 1 below.
| Gas (ref) | solubility (aq) (g/L) | solubility (mM) |
| CO2 | 1.7 | 38 |
| O2 | 0.044 | 1.3 |
| N2 | 0.019 | 0.68 |
Offer reasons that explain the significantly higher (but still low) solubility of CO2 in water compared to O2 and N2.
- Answer
-
How does the enzyme facilitate the transport of CO2 in blood? How does it maintain intracellular pH?
- Answer
-
We'll explore the structure of two different CAs, human carbonic anhydrase II and the carbonic anhydrase from Neisseria gonorrhea in this guided problem-solving module.
Human carbonic anhydrase II
The structure of native human carbonic anhydrase II and its catalytic mechanism is shown in Figure 7 below.
Figure 7: Structure of native human carbonic anhydrase II (Zn-CA II) and its catalytic mechanism. Kim, J.K., Lee, C., Lim, S.W. et al.Nat Commun 11, 4557 (2020). https://doi.org/10.1038/s41467-020-18425-5. Creative Commons Attribution 4.0 International License, http://creativecommons.org/licenses/by/4.0/.
Panel a shows the active site consists of the zincbinding site, hydrophobic/hydrophilic regions, and the entrance conduit (EC).
Panel b shows the water networks in the active site that are responsible for the proton transfer (red) and substrate/product/water exchange (blue) during enzyme catalysis.
Panel c shows the forward reaction mechanism of Zn-CA II.
The active site itself lies at the bottom of a deep cavity (15 Å deep) in the protein, which is readily accessible to solvent
An interactive iCn3D model of human carbonic anhydrase II with bound bicarbonate and CO2 (2VVB) is shown in Figure 8 below.
Figure 8: Human carbonic anhydrase II with bound bicarbonate and CO2 (2VVB) (Copyright; author via source).
Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...ibgmgsm3UWhjX6
The active site residues shown in Figure 8 are labeled and shown as sticks. Bound CO2 and HCO3- are also shown as sticks.
 This question addresses the Biomolecular Visualization Framework theme(s) Molecular Interactions (MI), Atomic Geometry (AG)
This question addresses the Biomolecular Visualization Framework theme(s) Molecular Interactions (MI), Atomic Geometry (AG)
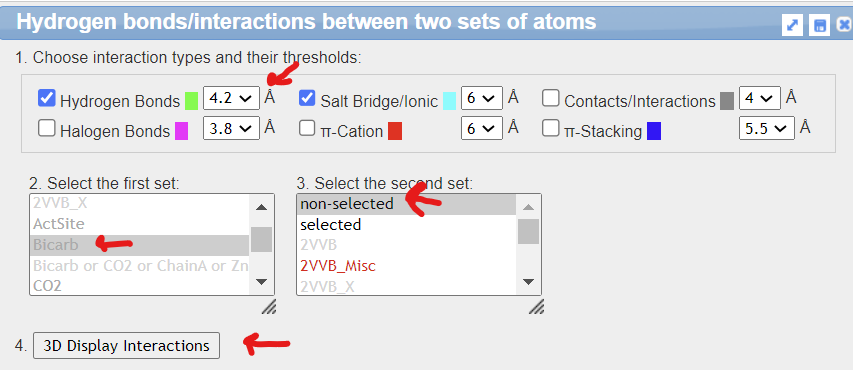
Using iCn3D to show the noncovalent interactions between bicarbonate, Zn, and the protein by using iCn3D. Measure the distance between the hydrogen-bonded atoms in HCO3- and Thr 199.
iCn3D instructions
Trackpad and Mouse Controls
•rotate: click and drag (mouse: left click and drag)
•zoom: pinch and spread (mouse: rotate the scroll wheel)
•translate: two-finger click and drag (mouse: right click and drag)
•Re-center: left click View from the top menu bar, then select “Center Selection”
•Note: ctrl-click on a PC = command-click on Mac; alt-click on PC = option click on Mac
- Open the external link: https://structure.ncbi.nlm.nih.gov/i...ibgmgsm3UWhjX6
- From the top menu bar, choose Analysis, Interactions
- In the new popup window select the following prompts, then click 4. 3D Display interactions
4. Close all but the main modeling window.
5. Zoom into the bicarbonate binding site. From the top menu bar, choose Analysis, Distance, distance between 2 atoms, and pick the two atoms (by holding down the Alt key or Option on a Mac) involved in the hydrogen bond between the bicarbonate and the amide
6. Rescale the label size by choosing Analysis, Label Scale, 0.4.
7. From the top menu bar choose Select, Toggle highlights to remove yellow and box highligting.
8. Save a PNG file by choosing
9. You can reload the PNG file directly into iCn3D by choosing File, Open File, iCn3D PNG image
- Answer
-
What is a likely function of Val, Leu and Trp cluster in CAII (shown in Figures 7 and 8)?
- Answer
-
What ligand would likely replace OH- at low pH values? What would happen to the activity of the enzyme at lower pHs?
- Answer
-
From the mechanism shown in Figure 7, does bicarbonate coordinate the Zn2+ ion in a monodentate or bidentate manner?
- Answer
-
Write a verbal description of the mechanism of CAII based on Figure 7
- Answer
-
Why is a proton transfer path needed?
- Answer
-
Thr 199 plays a key role in the mechanism. State a reason for its importance in the reversible reaction.
- Answer
-
The dissociation constant KD (or Kis) for bicarbonate binding to HCAII is about 77 mM. What kind of inhibitor might it be for the forward reaction?
- Answer
-
Using the equation below, at what ratio of CO2/[HCO3-] would the rate for the forward reaction (CO2 sequestration) be cut in half? Assume the VM=1, KM forward reaction is 1, [S] = 1 and Kis is 2 (a wide range of values are reported in the Brenda Database.
v0=VMSKM(1+IKis)+S
If you need some help, hover over -
Give me a hint!- Answer
-
The rate-limiting step for human CA II is the dissociation of a proton from Zn2+-bound water and not the removal of the resulting proton from the enzyme. What does that imply about the rate of removal of the proton from the enzyme?
- Answer
-
It must be very fast, that is at diffusion-controlled limits through the H-bond channel.
Synthetic mimetics of the active site of CA have been made. These are heteromacrocycles (similar to the heme of hemoglobin) as shown in Figure 9 below.
Figure 9:
The macrocycle mimetic has three imidazole groups coordinating zinc.
The macrocycle mimetic has three imidazole groups coordinating zinc. Is the bicarbonate coordinated to the Zn2+ in a monodentate or bidentate fashion? From the "denticities" of the interactions of bicarbonate and Zn2+ for human CAII and the mimetic, which catalyst, CAII or the macrocycle would you expect to have a lower KD for bicarbonate? How might this affect the rate-limiting step for the mimetic?
- Answer
-
What is the utility of having both CO2 and HCO3- bind weakly to the enzyme
- Answer
-
Carbonic anhydrase from Neisseria gonorrhea (ngCA)
Data from: Jo, B., Park, T., Park, H. et al. Engineering de novo disulfide bond in bacterial α-type carbonic anhydrase for thermostable carbon sequestration. Sci Rep 6, 29322 (2016). https://doi.org/10.1038/srep29322. Creative Commons Attribution 4.0 International License. http://creativecommons.org/licenses/by/4.0/
Now that we understand the general chemistry, structure, and reaction mechanism of carbon anhydrase (at least the alpha human CAII form), let's explore efforts to engineer more thermostable variants. One example is the carbonic anhydrase from N. gonorrheas. This CA has been used as a target for mutagenesis to increase thermal stability of the enzyme, through the introduction of new disulfide bonds.
Even though only about 35% of the amino acids are identical, the overall structures are similar. This is illustrated in Figure 10 below.
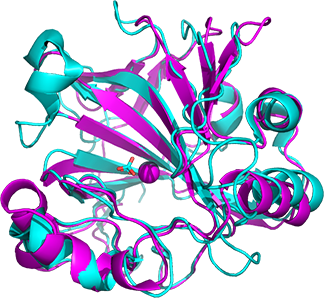
Figure 10: Alignment of the carbonic anhydrase from Neisseria gonorrhea (NG-CA) magenta,1KOQ) and human CA II (cyan, 2VVB)
The active site is mostly conserved compared to human CA II. The Zn2+ bound water has a pKa of around 6.5, compared to the value of 7.0 in human CA II. The hydrophobic patch (pocket) is similar, with Phe 93, Leu 153 and Tyr 72 in the NG-CA replacing Phe 95, Phe 176, Phe 70 in human CA II, respectively. The histidine ligands to Zn2+ are His92 (94), His94 (96), and His111 (119), where the numbers in parentheses represent Hu CA II. The proton removed from Zn2+ bound water is transferred to His 66 (64 in human CA II) and then to His 64.
The single disulfide bond between 181 and C28 is shown in Figure 1 below
Figure 11: Single disulfide bond between 181 and C28 in wild type Carbonic anhydrase from Neisseria gonorrhea
 This question addresses the Biomolecular Visualization Framework theme(s) Atomic Geometry (AG)
This question addresses the Biomolecular Visualization Framework theme(s) Atomic Geometry (AG)
Identify the correct torsion angles in Figure x above. Verbal definitions of torsional angles in a peptide chain are listed below. The successive atoms after the Cα leading away from the backbone atoms are Xβ-Xγ-Xδ-Xε (in that order). C is the backbone carbonyl C and N is the backbone nitrogen atom.
- phi (φ) is the angle of right-handed rotation around N-Cα bond. φ = 0 if the Cα-C bond is cis (eclipsed) to the C-N bond. Values range from -180 to 180 degrees.
- psi (ψ) is the angle of right-handed rotation around Cα -C bond. ψ = 0 if the C-N bond is cis (eclipsed) to the N-Cα bond. Values range from -180 to 180 degrees.
- chi1 (χ1) is the rotation around N-Cα-Xβ-Xγ
- chi2 (χ2) is the rotation around Cα-Xβ-Xγ-Xδ
- chi3 (χ3) is the rotation around Xβ-Xγ-Xδ-Xε
- Answer
-
 This question addresses the Biomolecular Visualization Framework theme(s) Atomic Geometry (AG), Topology and Connectivity (TC)
This question addresses the Biomolecular Visualization Framework theme(s) Atomic Geometry (AG), Topology and Connectivity (TC)
Figure 12 below shows an interactive iCn3D model of the atoms within 4A of the disulfide bond in Carbonic anhydrase from Neisseria gonorrhea (1KOQ). Rotate the model to determine the approximate chi3 (χ3) dihedral angle. Hint: site down the S-S bond.
Figure 12: Atoms within 4A of the disulfide bond in Carbonic anhydrase from Neisseria gonorrhea (1KOQ). Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...nutjP6EL2PubRA
- Answer
-
We mentioned previously that variants with higher thermal stability are likely to be more rigid and less flexible. Flexibility can be determined through analysis of molecular dynamic simulations and also by examining of the B factor values in PDB file. This number is a measure of the displacement of an atom from a mean The numbers in the last column in the file are called the temperature factors or B-factor. The B-factor describes the mean-square displacement, a measure of the displacement of an atom from an average value. If the atoms are more flexible, the electron density determined in x-ray structures is lower than if the atoms are more fixed, which gives high electron density.
To make stabilizing disulfide bonds, investigators found site chains close enough that when mutated to cysteines could potentially form disulfide bonds. In addition, they search for such residues in surface loops (without alpha and beta structure) which are inherently more flexible. Introducing disulfide bonds into the loop would stabilize it and make it more rigid. Table 2 below shows a description of the double cysteine CA variants in the study.
| Variant designation | Position | Wild-type residues | Loop length | Sum of B-factors |
|---|---|---|---|---|
| T133C/D197C | 133, 197 | Thr/Asp | 63 | 87.60 |
| P56C/P156C | 56, 156 | Pro/Pro | 99 | 80.82 |
| N63C/P145C | 63, 145 | Asn/Pro | 81 | 77.17 |
Table 2: Description of the double cysteine CA variants in ngCA
The locations of the side chair targeted for mutations to cysteine pairs are shown in Figure 13 below.
Figure 13: 3D structure of ngCA and location of residue pairs for disulfide engineering
The zinc (not shown)-coordinating histidine residues in the catalytic active site are shown in green. The proton shuttle histidine residue is shown in magenta. The native disulfide bond is colored yellow.
An interactive iCn3D model of carbonic anhydrase from Neisseria gonorrhea (1KOQ) highlighting the 3 pairs of sidechains for mutations is shown in Figure 14 below.
Figure 14: Carbonic anhydrase (Neisseria gonorrhea) with 3 paired side chains for engineered disulfide (1KOQ) (Copyright; author via source).
Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...KRP2tFJ1pV1FF6
The mutations were made and the wild-type proteins and three mutants were subjected to SDS-polyacrylamide gel electrophoresis (SDS-PAGE). The stained gels are shown in Figure 15 below.
Figure 15: Expression and purification of disulfide CA variants.
Panel (a) shows the expression of the protein in transformed cells 25 °C after IPTG induction and fractionated into soluble and insoluble fractions. SHuffle strain, an engineered E. coli strain that promotes cytoplasmic disulfide bond formation, was used.
Panel (b) shows purification results. Each lane was loaded with 4 μg of each purified CA variant. The proteins were visualized with Coomassie blue staining after SDS-PAGE. The arrow indicates the position of the bands corresponding to ngCA variants. Lane: M, molecular weight marker; S, soluble fraction; IS, insoluble fraction.
a. Interpret the results of the PAGE gels in Figure 15 above
b. How pure were the proteins based on the PAGE gel result in Panel (b). Can you infer from the gel that the proteins folded correctly?
- Answer
-
The investigators next determined if the expressed and purified wild-type and mutant proteins had the correct number of disulfide bonds. They did this by reacting the proteins in the absence and presence of dithiothreitol with DTNB or 5,5'-dithiobis(2-nitrobenzoic acid), also called Ellman's Reagent. Both structures are shown in Figure 16 below.
Figure 16: Structures of DTT and DTNB
DTT is a reducing agent that cleaves disulfide.
Draw a mechanism showing the reaction of a disulfide with DTT.
- Answer
-
Draw a mechanism showing the reaction of free sulfhydryl (like Cys) with Ellman's reagent
- Answer
-
The results of the reaction of the proteins with Ellmans's agent, in the presence and absence of DTT, are shown in Table 3 below.
| CA variant | Free thiol/protein (mol/mol) a | Deduced no. S-S bonds | |
|---|---|---|---|
| −DTT | +DTT | ||
| Wild-type | 0.06 ± 0.02 | 1.80 ± 0.14 | ? |
| T133C/D197C | 0.08 ± 0.02 | 3.79 ± 0.22 | ? |
| P56C/P156C | 0.06 ± 0.03 | 3.89 ± 0.06 | ? |
| N63C/P145C | 0.08 ± 0.03 | 3.75 ± 0.05 | ? |
Table 3: Analysis of disulfides in CA using Ellman's reagent. aNumbers are represented in mean ± SD.
How many S-S would you deduce from the table are present in the wild-type and mutant enzymes? Did the correct disulfide bonds form? Explain your answers
- Answer
-
Table 4 below shows the catalytic activities of the disulfide CA variants at 25 °C.
| CA variant | CO2 hydration activity | |||
|---|---|---|---|---|
| Relative esterase activity a | kcat × 10−4 (s−1) | K M (mM) | kcat /KM × 10−6(M−1 s−1) | |
| Wild-type | 1.00 | 1.44 | 14.2 | 1.01 |
| T133C/D197C | 1.49 | 1.97 | 16.7 | 1.18 |
| P56C/P156C | 1.03 | 1.44 | 16.9 | 0.85 |
| N63C/P145C | 0.55 | 0.27 | 17.3 | 0.16 |
Table 4: catalytic activities of the disulfide CA variants at 25 °C aThe specific activity of the wild-type corresponds to 0.22 U/μmol-enzyme.
Why did the investigators conduct this experiment? Interpret the results
- Answer
-
Now comes the big question: were the investigators able to engineer thermal stability into the carbonic anhydrase? Experimental results to show the thermostability of the disulfide CA mutants are shown in Figure 17 below.
Figure 17: Thermostability of the disulfide CA variants
Panel (a) shows short-term kinetic stability. The enzyme solutions (40 μM) were incubated for 30 min at different temperatures, and the residual activities were measured by esterase activity assay. Activities of 100% correspond to untreated samples. Panel (b) shows long-term kinetic stability at 70 °C. The half-lives (t1/2) of the CA variants were estimated by fitting the experimental data to an exponential decay curve. Each value represents the mean of at least three independent experiments, and the error bars represent the standard deviations.
Analyze the results in Panels (a) an (b). Which protein was most thermostable over the short (30 minute) and long (hours) incubating time at elevated temperatures?
- Answer
-
Panel (c) shows heat-induced denaturation of disulfide CA variants. Temperature-dependent changes of the circular dichroism ellipticity were recorded at 220 nm on CD spectrometer. The denaturation curves were normalized to the fraction of unfolded protein. The horizontal dashed line indicates the point at which the fraction of unfolded protein is 0.5. The vertical dashed lines point to TM values. Panel (d) shows the overall RMSD of disulfide variants from molecular dynamic simulations performed at 400 K for 20 ns.
Analyze the results in Panel (c). What do changes in the CD helicity show? Which protein was most thermostable based on TM values? Is the decrease in enzyme activity in panels (a) and (b) result from the denaturation of the protein?
- Answer
-
Analyze the molecular dynamics simulation results in Panel (d)
- Answer
-
You may remember from both introductory chemistry and from Chapter 4.12, that you can calculate the thermodynamic parameters, ΔHo and ΔSo for N ↔D at room temperature from thermal denaturation curves using the van 't Hoff equation.
In this case, Keq values can be calculated from thermal denaturation curves by monitoring change in CD signal at 220 nm, and applying this equation (also from Chapter 3.12).
Keq=[D]eq[N]eq=fDfN=fD1−fD
From this, we can calculate ΔG0.
ΔG0=−RTlnKeq=−RTln[fD1−fD]
Knowing Keq, ΔH0, DS0 can be calculated as shown below. A semi-log plot of lnKeq vs 1/T is a straight line with a slope of - ΔH0R and a y-intercept of + ΔS0/R, where R is the ideal gas constant.
ΔG0=ΔH0−TΔS0=−RTlnKeqlnKeq=−ΔH0−TΔS0RTlnKeq=−ΔH0RT+ΔS0R
The equation below shows that the derivative of equation (8) with respect to 1/T (i.e. the slope of equation 8 plotted as lnKeq vs 1/T) is indeed -ΔH0/R. Equation (9) is the van 't Hoff equation, and the calculated value of the enthalpy change is termed the van 't Hoff enthalpy, ΔH0vHoff.
dlnKeqd(1/T)=−ΔH0R=−ΔH0vHoffR
Using this method, the thermodynamic parameters for unfolding of the protein were calculated. The results are shown in Table 4 below.
| CA variant | Melting temperature, TM (°C) | Enthalpy change of unfolding, ΔH (kcal mol−1) | Entropy change of unfolding, ΔS (kcal mol−1 K−1) |
|---|---|---|---|
| Wild-type | 73.6 | 48.8 | 0.141 |
| T133C/D197C | 74.7 | 52.8 | 0.153 |
| P56C/P156C | 77.4 | 35.1 | 0.091 |
| N63C/P145C | 81.4 | 30.0 | 0.085 |
Table 4: Thermodynamic parameters for protein unfolding for WT and mutant CAs
Which effects, enthalpy or entropy of unfolding, were associated with the increased thermal stability of the mutants compared to the wild-type protein. Remember were are considering the denaturation reaction, N↔ D.
- Answer
-
Finally, the enzymatic activity of the wild-type and mutants CAs (using a small ester substrate) were studied as a function of temperature. The relative activity of the wild-type and all 3 disulfide mutants are plotted as a function of temperature in the histogram graphs shown in Figure 18 below.
Figure 18: Effect of temperature on the activity of disulfide CA variants.
Esterase activities of disulfide variants were measured at each temperature and normalized to the activity of each enzyme at 25 °C. Each value represents the mean of three independent experiments, and the error bars represent the standard deviations. If you plotted the data as curves, you would get bell-shaped graphs.
Explain why the histogram plots (and line plots if they were drawn) are bell-shaped. Are the results in accordance with the previous results.
- Answer
-
Disulfide engineering
Craig, D.B., Dombkowski, A.A. Disulfide by Design 2.0: a web-based tool for disulfide engineering in proteins. BMC Bioinformatics 14, 346 (2013). https://doi.org/10.1186/1471-2105-14-346. Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
Several computational programs have been developed to determine amino acid pairs that could be mutated to high-temperature stabilizing disulfide bonds. The stabilizing effects appear largest when the disulfide bond is made within the largest (and most flexible) loops (between 25-75 residues). These loops also had the highest residue B-factors.
In selecting pairs to form engineered disulfide, not only proximity (distance) but also geometry (torsion angles) of the resulting disulfide bond are important. We saw this previously in the analysis of the energy of butane rotamers, as illustrated in Figure 19 below.
Figure 19: Newman projections for butane
Programs to determine amino acid pairs to mutate for disulfide bond formation test S-S bond torsional stability by determining the torsion angle χ3 for the S-S bond. Evaluation of a database of many native proteins shows the χ3 angle are centered in two major peaks at -87 and +97 degrees, as shown in Figure 20 below
Figure 30: Distribution of χ 3 torsion angles observed in 1505 native disulfide bonds found in 331 PDB protein structures. Peaks occur at -87 and +97 degrees. Craig, D.B., Dombkowski, A.A. Disulfide by Design 2.0: a web-based tool for disulfide engineering in proteins. BMC Bioinformatics 14, 346 (2013). https://doi.org/10.1186/1471-2105-14-346. Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0),
Does your calculated value of χ3 for the native disulfide in carbonic anhydrase from Neisseria gonorrhea follow the observed angles?
- Answer
-
An empirical energy equation was developed to determine the E vs χ3 dihedral angle for disulfide bonds (Craig, D.B., Dombkowski, A.A., ibid). It is shown below.
E(χ3)=4.0[1−cos(1.957[χ3+87])]
A graph of the equation made with Excel is shown in Figure 21 below
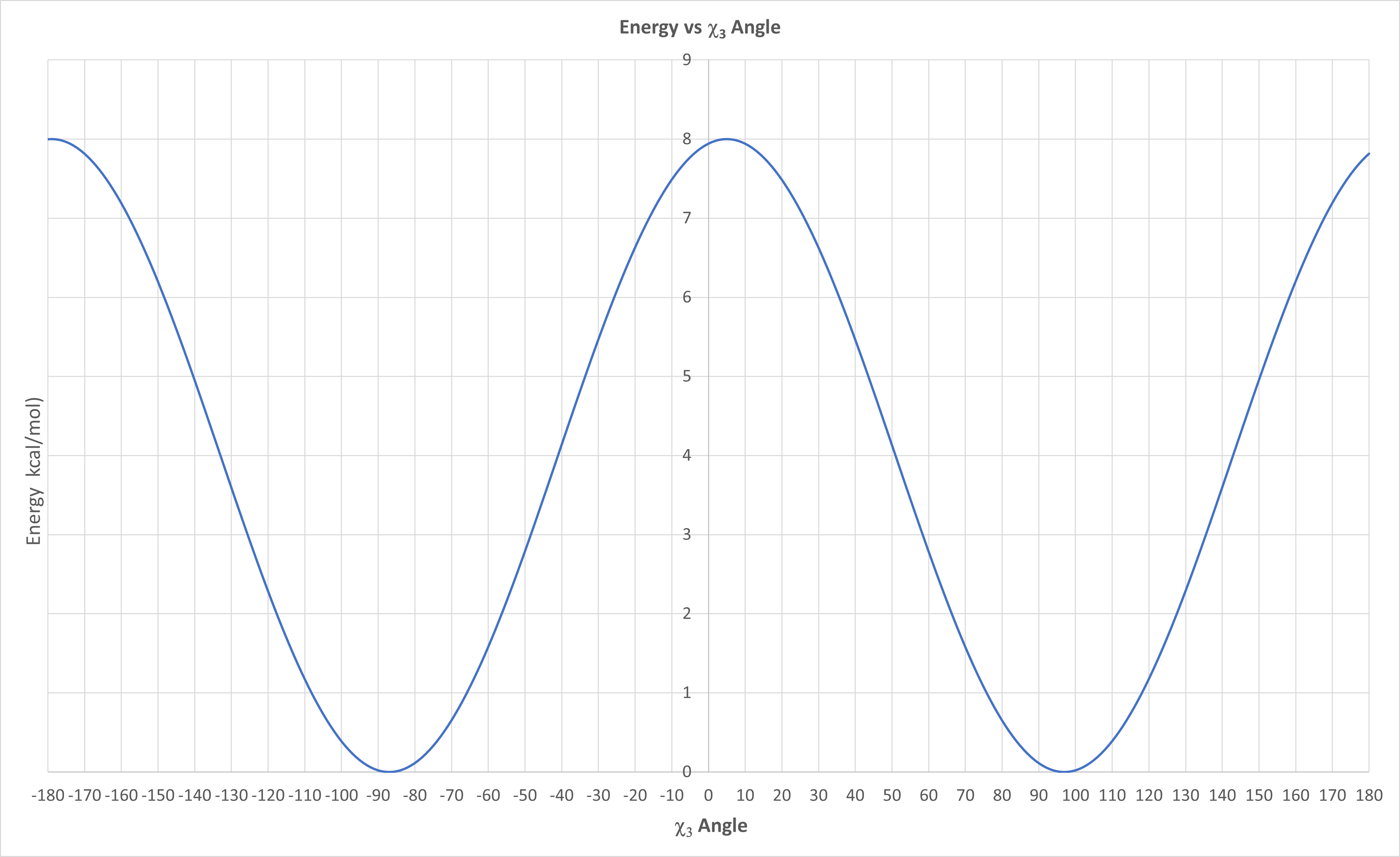
Figure 21: E vs χ3 dihedral angle for disulfide bonds from empirical equation
Figure 22 shows the distribution of energy values in the 1505 native disulfide bonds using our updated function. The study by Craig and Dombkowski showed that almost all (90%) of disulfides in native proteins in the PDB have an energy < 2.2 kcal/mol, so this metric could be used to determine possible disulfide bond pairs created by mutagenesis.
Figure 22: Distribution of the disulfide bond energy calculated for 1505 native disulfide bonds in our survey set using the DbD2 energy function. The mean value is 1.0 kcal/mol, and the 90th percentile is 2.2 kcal/mol. (Craig, D.B., Dombkowski, A.A., ibid)
You calculated the approximate value for χ3 dihedral for the C28-C181 native disulfide bond form in Carbonic anhydrase from Neisseria gonorrhea using iCn3D in Question x above. Determine the approximate energy from the empirical function graph in Figure x above for that χ3 dihedral. What percent of native disulfide bonds have that particular calculated energy in the 1505 native disulfide surveyed?
- Answer
-
Yet there are other important parameters as well that would affect the energy of the disulfide bond in the protein. Figure 23 below shows a comparison of native residue B-factors in stabilizing and destabilizing engineered disulfide bonds
Figure 23: Comparison of native residue B-factors in stabilizing and destabilizing engineered disulfide bonds. The native structures associated with engineered disulfides previously reported as stabilizing (S) or destabilizing (D), based on experimental evidence, were analyzed with DbD2. The mean B-factor for residues involved in stabilizing disulfide bonds was 31.6 compared with 16.5 for those involved in destabilizing bonds, P = 0.066. (Craig, D.B., Dombkowski, A.A., ibid)
Which proteins were more stabilized by engineered disulfide, those with higher or low B factors.
- Answer
-
Another Paper:
Prediction of disulfide bond engineering sites using a machine learning method
https://www.nature.com/articles/s41598-020-67230-z
Gao, X., Dong, X., Li, X. et al. Prediction of disulfide bond engineering sites using a machine learning method. Sci Rep 10, 10330 (2020). https://doi.org/10.1038/s41598-020-67230-z. Creative Commons Attribution 4.0 International License. http://creativecommons.org/licenses/by/4.0/
The amount of data present in a single PDB file is very large, but it is nothing compared to the collective data in all PDB files. If only we could extract empirical rules from the collective PDB files that govern disulfide bond formation. It turns out we can with machine learning and artificial intelligence that can be used to develop and train predictive algorithms. Machine learning has been used to predict amino acid pairs for cysteine mutations to form engineered disulfide bonds. It recognizes 99% of natural disulfide bonds. residues. It uses these parameters:
- distances between the alpha-carbons and the beta-carbons of the bonded cysteine residues
- three torsion angles around the disulfide bonds (χ1,χss,χ1’).
An example of one variable that helps define the stability of disulfide bonds is the distances between the Cα atoms for the disulfide-bonded cysteines, as shown in Figure 24 below.
Figure 24: The histogram of distances between Cα atoms of disulfide-bonded cysteines.
The distances range from 3.0 Å and 7.5 Å. Knowing this would constrain the number of choices for paired amino acid side chains for mutagenesis to produce disulfide.
Machine learning can also be used to find other distance constraints to optimize mutagensis experiments. Figure 25 below shows a graph of 10 different distances and their relative importance in determining disulfide bond stability.
Figure 25: The relevance of the distance features to the classification outcome. Out of the 45 unique distances, 20 distances have negligible influence on the classification performance. The distances between Cβ and main-chain atoms of the pairing residue are important features in disulfide bond classifications.
An interactive iCn3D model of carbonic anhydrase from Neisseria gonorrhea (1KOQ) highlighting two pairs of amino acids identified by machine learning as candidates for mutations to disulfide-bonded cysteines is shown in Figure 26 below.
Figure 26: Carbonic anhydrase from Neisseria gonorrhea (1KOQ) highlighting two pairs of amino acids identified by machine learning as candidates for mutations to disulfide-bonded cysteines (Copyright; author via source). Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...ZH6Tv1urwkKhz8
 This question addresses the Biomolecular Visualization Framework theme(s) Atomic Geometry (AG), Macromolecular Building Blocks (MB)
This question addresses the Biomolecular Visualization Framework theme(s) Atomic Geometry (AG), Macromolecular Building Blocks (MB)
Using the data in Figure 25 and measurements made using the iCn3D model above (Figure 25) , determine which pair would be most likely engineered into a disulfide bond. Complete the table below with the distances you made using iCn3D.
iCn3D instructions: Open the external link and follow these instructions
Trackpad and Mouse Controls
•rotate: click and drag (mouse: left click and drag)
•zoom: pinch and spread (mouse: rotate the scroll wheel)
•translate: two-finger click and drag (mouse: right click and drag)
•Re-center: left click View from the top menu bar, then select “Center Selection”
•Note: ctrl-click on a PC = command-click on Mac; alt-click on PC = option click on Mac
Instructions
- Zoom to clearly see the amino acid pair for distance measures
- From the top menu bar, choose Analysis, Distance, distance between 2 atoms
- Pick the appropriate 2 atoms for measure distance by holding down the Alt key and selecting both
- Record the distances in the table below.
| Mutation Pair | CB1-CA2 (Å) | CB1-CB2 (Å) | CA1-CB2 (Å) |
| 1 (L137) - 2 (W141) | |||
| 1 (Y54) - 1 (S160) |
- Answer
Additional References
- Himadri Bose and Tulasi Satyanarayana. Microbial Carbonic Anhydrases in Biomimetic Carbon Sequestration for Mitigating Global Warming: Prospects and Perspectives. Front Microbiol. 2017; 8: 1615. doi: 10.3389/fmicb.2017.01615
- Ayanne de Oliveira Maciel, Paul Christakopoulos, Ulrika Rova, Io Antonopoulou. Carbonic anhydrase to boost CO2 sequestration: Improving carbon capture utilization and storage (CCUS), Chemosphere, 299, 2022, https://doi.org/10.1016/j.chemosphere.2022.134419
- Craig Bettenhausen. The life-or-death race to improve carbon capture. Craig Bettenhausen. CEN. July 18, 2021
- Tanvi Sharma, Swati Sharma, Hesam Kamyab, Ashok Kumar Energizing the CO2 utilization by chemo-enzymatic approaches and potentiality of carbonic anhydrases: A review. Journal of Cleaner Production. Volume 247, 20 February 2020, 119138. https://doi.org/10.1016/j.jclepro.2019.119138
- Mustaffa, N.I.H.; Latif, M.T.; Wurl, O. The Role of Extracellular Carbonic Anhydrase in Biogeochemical Cycling: Recent Advances and Climate Change Responses. Int. J. Mol. Sci. 2021, 22, 7413. https://doi.org/10.3390/ijms22147413.
- Jo, B., Park, T., Park, H. et al. Engineering de novo disulfide bond in bacterial α-type carbonic anhydrase for thermostable carbon sequestration. Sci Rep 6, 29322 (2016). https://doi.org/10.1038/srep29322




.png?revision=1&size=bestfit&width=216&height=251)
.png?revision=1&size=bestfit&width=437&height=384)
.png?revision=1&size=bestfit&width=360)
V2.png?revision=1&size=bestfit&width=342&height=340)