12.4: Biological Oxidation-Reduction Reactions
- Page ID
- 15000
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)General Oxidizing Agents
Before we consider common biological oxidizing agents, lets look back at ones you saw in other chemistry classes. Oxidizing agents are required to oxidize organic molecules. In organic lab, you never used dioxygen as an oxidizing agent. It is difficult to limit the extent of oxidation using dioxygen. In addition, side reactions are likely given the nature of the reactive oxygen reduction products. The mechanisms of combustion reactions of organic molecules with dioxygen to produce carbon dioxide and water are very complicated. Table \(\PageIndex{1}\) belows some key steps in the combustion of methane.
Initiation
\[\ce{CH4 → CH3^{.} + H^{.}} \nonumber\]
\[\ce{O2 → 2 O^{.}} \nonumber\]
Propagation
\[\ce{CH4 + H^{.} → CH3^{.} + H2} \nonumber\]
\[\ce{CH4 + HO^{.} → CH3^{.} + H2O} \nonumber\]
\[\ce{CH3^{.} + O^{.} → CH2O + H^{.}} \nonumber\]
\[\ce{CH2O + HO^{.} → CHO^{.} + H2O} \nonumber\]
\[\ce{CH2O + H^{.} → CHO^{.} + H2} \nonumber\]
\[\ce{CHO^{.} → CO + H^{.}} \nonumber\]
\[\ce{CO + HO^{.} → CO2 + H^{.}} \nonumber\]
Branching
\[\ce{H^{.} + O2 → HO^{.} + O^{.}} \nonumber\]
Termination
\[\ce{H. + R^{.} + M → RH + M^{*}} \nonumber\]
In chemistry, other oxidizing agents are often used, including permanganate and chromate. Their mechanism of action is illustrated in Figure \(\PageIndex{1}\).
Oxygen can often be inserted into a molecule in a nonoxidative process by hydration of an alkene to an alcohol (a readily reversible reaction), which could then be oxidized to either an aldehyde/ketone or carboxylic acid using an appropriate oxidizing agent.
Most biological oxidation reactions (such as those found in glycolysis, Kreb Cycle, and fatty acid oxidation) do not use dioxygen as the immediate oxidizing agent. Rather they use nicotinamide adenine dinucleotide (NAD+) or flavin adenine dinucleotide (FAD) as oxidizing agents, which get reduced. Enzymes that uses these oxidizing agents are usually called dehydrogenases. Dioxygen can also be used to introduce oxygen atoms into biological molecules in oxidative reactions. Enzymes that introduce one oxygen atom of dioxygen into a molecule (and the other oxygen into water) are called monooxygenases. (Note: some monooxygenase that hydroxylate biomolecules are called hydroxylases.) Those that introduce both atoms of dioxygen into a substrate are called dioxygenases. These oxygenases are not usually used to oxidize organic molecules for energy production. Rather they introduce O atoms for other reasons, including increasing the solubility of nonpolar aromatics to facilitate secretion, and to produce new molecular species which have different biological activities. Finally, biological molecules can be oxidized by dioxygen in which no atoms of oxygen are added to the substrate. Rather, electrons lost from the oxidized substrate are passed via intermediate electron carriers to dioxygen , which get reduced to superoxide (if one electron is added), hydrogen peroxide (if two electrons are added) or water (if 4 electrons are added). These enzymes are called oxidases. (Note: The letters oxi- or oxygen- are used in all the enzymes that use dioxygen as the oxidizing agent.)
In this chapter section, we will discuss biological oxidation reactions. Most introductory biochemistry texts don't approach oxidation reactions in one cohesive chapter. Probably because of that, when I was learning biochemistry, I found the presentation of these different enzymes involved in redox reactions to be very confusing. Hopefully this section will alleviate that problem. First the chemistry of NAD+ and FAD will be discussed. Then the enzymes using dioxygen in oxidative reactions (monooxygenases, dioxygenases, and oxidases) will be explored.
The Chemistry of NAD+ and FAD
NAD+ is a derivative of nicotinic acid or nicotinamide, as illustrated in Figure \(\PageIndex{2}\).
Figure \(\PageIndex{2}\): Structure of niacin derivatives
It and its reduction product, NADH, exists in the cells as interconvertible members of a pool whose total concentration does not vary significantly with time. Hence, if carbohydrates and lipids are being oxidized by NAD+ to produce energy in the form of ATP, levels of NAD+ would begin to fall as NADH rises. A mechanism must be be present to regenerate NAD+ from NADH if oxidation is to continue. As we will see later, this happens in the muscle under anaerobic conditions (if dioxygen is lacking as when you are running a 100 or 200 m race, or if you are being chased by a saber-toothed tiger) when pyruvate + NADH react to form lactate + NAD+. The reaction is shown in Figure \(\PageIndex{3}\).
Figure \(\PageIndex{3}\): Conversion of pyruvate to lactate
Under aerobic conditions (sufficient dioxygen available), NADH is reoxidized in the mitochondria by electron transport through a variety of mobile electron carriers, which pass electrons to dioxygen (using the enzyme complex cytochrome C oxidase) to form water.
NAD+/NADH can undergo two electron redox steps, in which a hydride is transferred from an organic molecule to the NAD+, with the electrons flowing to the positively charged nitrogen of NAD+ which serves as an electron sink. NADH does not react well with dioxygen, since single electron transfers to/from NAD+/NADH produce free radical species which can not be stabilized effectively. All NAD+/NADH reactions in the body appear to involve 2 electron hydride transfers. Figure \(\PageIndex{5}\) shows both 1 and 2 electrons to NAD+.
Figure \(\PageIndex{4}\):
Figure \(\PageIndex{4}\): 1 and 2 electrons to NAD+
FAD (or flavin mononucleotide-FMN) and its reduction product, FADH2, are derivatives of riboflavin, as shown in Figure \(\PageIndex{5}\).
Figure \(\PageIndex{5}\): Structures of riboflavin, FMN and FAD
FAD/FADH2 differ from NAD+/NADH since they are bound tightly (KD approx 10-7 - 10-11 M) to enzymes which use them. This is because FADH2 is susceptible to reactions with dioxygen, since FAD/FADH2 can form stable free radicals arising from single electron transfers. FAD/FADH2 can undergo 1 OR 2 electrons transfers. This is illustrated in Figure \(\PageIndex{6}\).
Figure \(\PageIndex{6}\): 1 and 2 electrons reduction of FAD
FAD/FADH2 are tightly bound to enzymes so as to control the nature of the oxidizing/reducing agents that interact with them. (i.e. so dioxygen in the cell won't react with them in the cytoplasm.) If bound FAD is used to oxidize a substrate, the enzyme would be inactive in any further catalytic steps unless the bound FADH2 is reoxidized by another oxidizing agent.
Dehydrogenases
These enzymes use NAD+/NADH or FAD/FADH2 and are named for the substrate that is oxidized. For instance in the reaction:
\[\ce{pyruvate + NADH <=> lactate + NAD^{+}} \nonumber\]
which is used to regenerate NAD+ under anerobic conditions, the enzyme is named lactate dehydrogenase. As in acid/base reactions, when the preferred direction for the reaction (from a ΔGo perspective) is from stronger acid to weaker (conjugate) acid, the preferred direction for a redox reaction is in the direction from strong to weak oxidizing/reducing agents. This can easily be determined from charts of standard reduction potentials, and using the equation: ΔGo = -nFEo,
- where F is the Faraday constant (96,494 Coulombs/mol e- = 96, 494 J/(V.mol) = 23.06 kcal/(V.mol) or 96 kJ/(V.mol). One Faraday is the charge per one mol of electrons).
- and Eo, the standard EMF or standard cell potential (total voltage at standard state conditions), which can be determined by adding the standard reduction potentials (Eo) for the two appropriate half-reactions, after reversing the equation for the half-reaction that represents the oxidation. Hence Eo=Eoreduction−Eooxidation.
When n=2 (number of electrons) which is common for oxidations of organic molecules,
ΔGo (kcal/mol) = - 46.12Eo or approximately -50E0 (or -193Eo kJ/mol)
Notice when Eo > 0, ΔGo < 0, the reaction as written is favored under standard conditions. Note in the table below that many of the half reactions involve protons. For biological reactions involving free protons, the standard state concentration for the protons are not 1 M as for other solutes in solution, but defined to be the hydronium ion concentration at pH 7.0. The Eo and ΔGo values for the reactions involving hydrogen ions at a standard state of pH 7.0 are usually written as Eo' and ΔGo'.
Common standard reduction potentials are shown in Table \(\PageIndex{2}\) below.
| oxidant | reductant | n (electrons) | Eo' (volts), 25oC |
|---|---|---|---|
| Acetate + carbon dioxide | pyruvate | 2 | -0.70 |
| succinate + CO2 + 2H+ | α−ketoglutarate + H2O | 2 | -0.67 |
| acetate | acetaldehyde | 2 | -0.60 |
| glycerate-3-P | glyceraldehyde-3-P + H2O | 2 | -0.55 |
| O2 | O2- | 1 | -0.45 |
| ferredoxin (ox) | ferredoxin (red) | 1 | -0.43 |
| Carbon dioxide | formate | 2 | -0.42 |
| 2H+ | H2 | 2 | -0.42 |
| α-ketoglutarate + CO2 + 2H+ | isocitrate | 2 | -0.38 |
| acetoacetate | β-hydroxybutyrate | 2 | -0.35 |
| Cystine | cysteine | 2 | -0.34 |
| Pyruvate + CO2 | malate | 2 | -0.33 |
| NAD+ + 2H+ | NADH + H+ | 2 | -0.32 |
| NADP+ + 2H+ | NADPH + H+ | 2 | -0.32 |
| FMN (enzyme bound) | FMNH2 | 2 | -0.30 |
| Lipoic acid, ox | Lipoic acid, red | 2 | -0.29 |
| 1,3 bisphosphoglycerate + 2H+ | glyceraldehyde-3-P + Pi | 2 | -0.29 |
| Glutathione, ox | Glutathione, red | 2 | -0.23 |
| FAD (free) + 2H+ | FADH2 | 2 | -0.22 |
| Acetaldehyde + 2H+ | ethanol | 2 | -0.20 |
| Pyruvate + 2H+ | lactate | 2 | -0.19 |
| Oxalacetate + 2H+ | malate | 2 | -0.17 |
| α-ketoglutarate + NH4+ | glutamate | 2 | -0.14 |
| FAD + 2H+ (bound) | FADH2 (bound) | 2 | 0.003-0.09 |
| Methylene blue, ox | Methylene blue, red | 2 | 0.01 |
| Fumarate + 2H+ | succinate | 2 | 0.03 |
| CoQ (Ubiquinone - UQ) + H+ | UQH | 1 | 0.031 |
| UQ + 2H+ | UQH2 | 2 | 0.06 |
| Dehydroascorbic acid | ascorbic acid | 2 | 0.06 |
| Ubiquinone; ox | red | 2 | 0.10 |
| Cytochrome b2; Fe3+ | Cytochrome b2; Fe2+ | 1 | 0.12 |
| Cytochrome c1; Fe3+ | Cytochrome c1; Fe2+ | 1 | 0.22 |
| Cytochrome c; Fe3+ | Cytochrome c; Fe2+ | 1 | 0.25 |
| Cytochrome a; Fe3+ | Cytochrome a; Fe2+ | 1 | 0.29 |
| 1/2 O2 + H2O | H2O2 | 2 | 0.30 |
| Cytochrome a3; Fe3+ | Cytochrome a3; Fe2+ | 1 | 0.35 |
| Ferricyanide | ferrocyanide | 2 | 0.36 |
| Cytochrome f; Fe3+ | Cytochrome f; Fe2+ | 1 | 0.37 |
| Nitrate | nitrite | 1 | 0.42 |
| Photosystem P700 | . | . | 0.43 |
| Fe3+ | Fe2+ | 1 | 0.77 |
| 1/2 O2 + 2H+ | H2O | 2 | 0.816 |
The mechanism for the oxidation of a substrate by NAD+ involves concerted hydride transfer to one face of NAD+. Hydride transfer is possible since water is excluded from the active site. It is facilitated by removal of a proton from an oxygen on an alcoholic substituent, for example, adjacent to the departing hydride. The negative charge on the oxide acts as a "source" of electrons, which can then flow through the hydride transfer to the positively charged ring nitrogen of NAD+, which acts as an electron "sink". This is crudely illustrated in the cartoon shown in Figure \(\PageIndex{7}\), which shows the oxidation of ethanol by the enzyme alcohol dehydrogenase.
For substrates like ethanol that lose a hydride from a methylene carbon atom that has two hydrogens, only one of them is lost (either the proR or proS) from the prochiral center.
The site on NAD+ that receives the hydride, as well as the entire ring, is planar with sp2 hybridization. When bound to the enzyme, the hydride is transfer to the Re face of he ring. The same occurs in the reverse reaction when the hydride from NADH is transferred to the re face of acetaldehyde. Re and Si faces can be determined using by prioritizing the substituents attached to the sp2 carbon using the Cahn-Ingold rules. The reversible transfer of the proR hydrogens to the Re faces of the reactants in the reversible conversion of ethanol to acetaldehyde are shown in Figure \(\PageIndex{8}\).
Figure \(\PageIndex{8}\): Reversible transfer of the proR hydrogens to the Re faces of the reactants in the alcohol dehydrogenase reaction
FAD has a more positive reduction potential than NAD+ so it is used for more "demanding" oxidation reactions, such as dehydrogenation of a C-C bond to form an alkene. You will notice on standard reduction potential tables that the potential of FAD is often listed several times and depends on the enzyme. This is because the FAD is tightly bound to the enzyme so its tendency to acquire electrons depends on its environment, in much the same fashion as the pKa of an amino acid side chain (which reflects is tendency to release protons) is affected by the environment of the amino acid side chain in the protein. The standard reduction potential for flavin enzymes varies from -465 mV to + 149 mV. Compare this to the reduction potential of free FAD/FADH2, which in aqueous solution is -208 mV. The standard reduction potential of the flavin in D-amino acid oxidase, a flavoprotein, is about 0.0 V. Remember, the more positive the standard reduction potential, the more likely the reactant will be reduced and hence act as an oxidizing agent. Hence the FAD in D-amino acid oxidase is a better oxidizing agent than free FAD. The KD for binding of FAD to the enzyme is 10-7 M compared to the KD for binding of FADH2, which is 10-14 M. By gaining electrons, the flavin binds more tightly, which preferentially stabilizes the bound FADH2 compared to the bound FAD. This shifts the equilibrium of FAD ↔ FADH2 to the right, making the bound FAD a stronger oxidizing agent.
A mechanism for the 2-electron hydride reduction of FAD is shown in Figure 6 above.
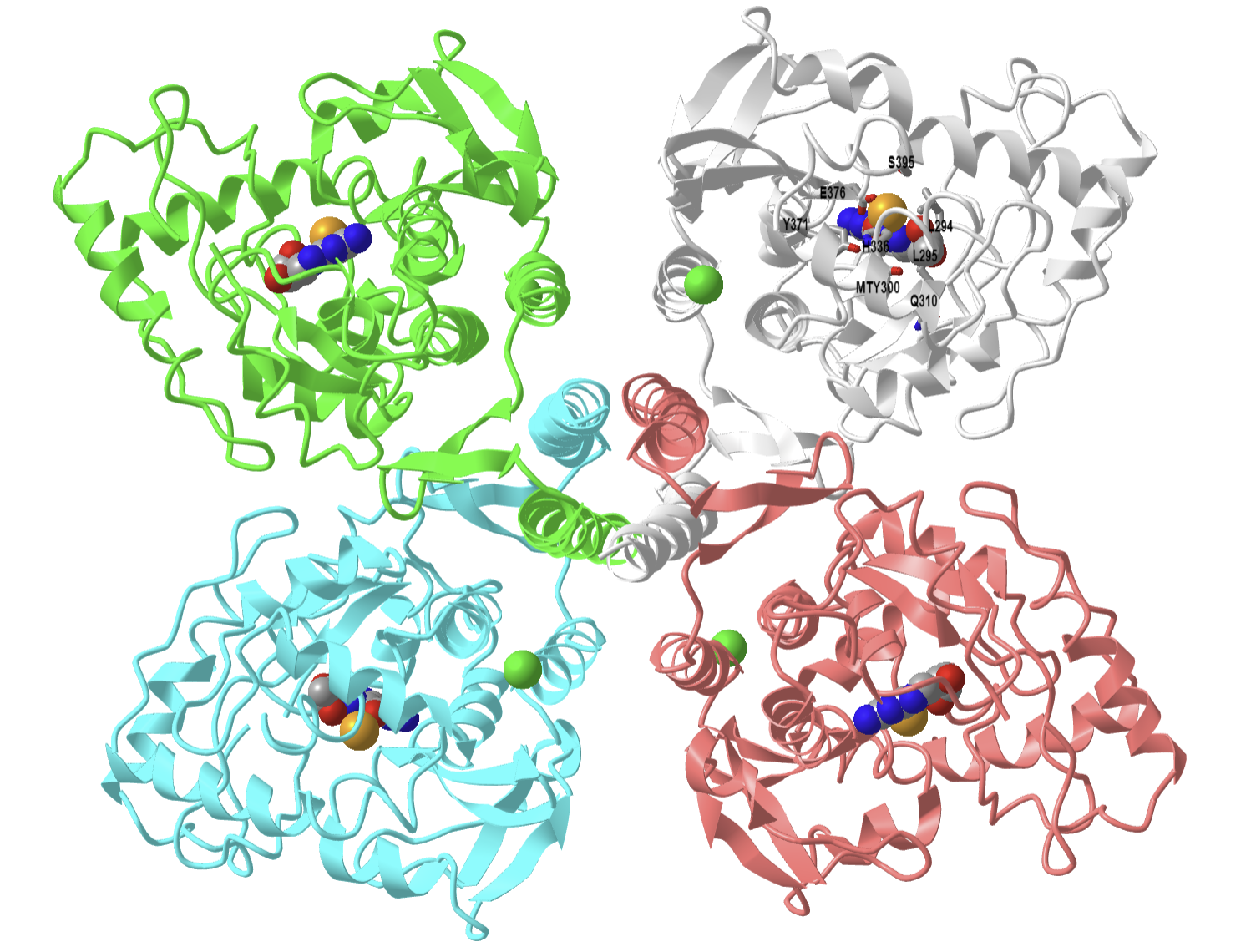
Figure \(\PageIndex{9}\) below shows an interactive iCn3D model of D-amino acid oxidase bound to FAD and a trifluoroalanine (1C0L) .
Figure \(\PageIndex{9}\): D-amino acid oxidase bound to FAD and a trifluoroalanine (1C0L). (Copyright; author via source). Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...wYfRAK9TBSt2c8
FAD is shown in spacefill and colored yellow. Only the amino acids interacting with FAD are shown as a surface with underlying CPK colored sticks. The noncovalent interactions are shown as sticks. Note that you rotate the molecule, you will see the the FAD is almost completely buried which, along withe the extensive interactions with protein contributes to its low KD.
Thermodynamically, FAD, especially when covalently or tightly bound (and not released) from an enzyme, has a greater drive to be reduced so it is a stronger oxidizing agent than NAD+. Hence it will be used biologically for more difficult oxidation reactions. Converting C-C to C=C is thermodynamically more difficult than converting C-OH to C=O. That can likewise be inferred from standard reduction potential (SRP) values for organic molecules. For the reduction of alkenes to alkanes, the SRP generally varies from about +1 to +2.5V. For the reduction of an aldehyde to an alcohol, the SRP generally varies from about -2.5 to about 0V.
Hence the reduction of an alkene to an alkane is more favored than an aldehyde to an alcohol. So accordingly, the oxidation of an alkane to an alkene (reverse rx) is less favored than the oxidation of an alcohol to an aldehyde. This makes some intuitive sense since the alcohol is already partially oxidized. So biologically, the tough oxidations use FAD as the oxidizing agent. Of course, one of the toughest, the oxidation of H2O to O2 in photosynthesis, requires an even stronger oxidizing agent than O2 (of course). It is the oxygen-evolving complex, which we will see in the chapter on photosynthesis.
Can the standard reduction potential of a redox active center in a protein be tuned by changing the environment of that center, much as the pKa of an acid side chain can by changing the polarity of the environment? The answer is yes. The active site of azurin, a cupredoxin, has a redox active copper ion coordinated by a Cys and two His residues in a trigonal planar fashion. Met 121 serves as a weak axial ligand. Marshall et al. have reported a feasible method to manipulate the redox potential (Eo) of this active site. The wild type azurin was mutated to alter the hydrophobicity and hydrogen bonding capabilities, while maintaining the overall architecture of the metal binding site. Ser 46 was selected for mutation since it occupied a position similar to Asn in another cupredoxin that was involved in an important H bond binding two ligand binding loops. An N47S-mutation, which strengthened the hydrogen bond between the two ligand-containing loops increased Eo by ~130 mV while preserving metal binding site architecture as determined by UV-Vis spectroscopy. They also compared a M121Q mutant with wild-type M121 and with a M121L mutant. A plot of Eo vs log partition coefficient for transfer of the side chain from water to octanol was essentially linear with a positive slope, showing that the standard reduction potential depended on the hydrophobicity of the weakly coordinating ligand in the metal binding region. This behavior extended to double mutants (where one set of mutants involved M121). The investigators were able to tune the Eo over a 700 mv range!
Monooxygenases
An examples of monooxygenases are the hydroxylases which hydroxylate amino acids like tryptophane and tyrosine to form 5-hydroxytryptophan and 3-4-dihydroxyphenylalanine or dopa, respectively. These latter two substances can be decarboxylated using PLP-dependent enzymes to form the neurotransmitters 5 hydroxytryptamine (5HT or serotonin) and dopamine. The latter can be hydroxylated again to form norepinephrine, and subsequently methylated to form epinephrine. LSD and amphetamine are analogs of serotonin and dopamine, respectively. The derivative of tryptophan and tyrosine are shown in Figure \(\PageIndex{10}\).
Figure \(\PageIndex{10}\): Derivative of tryptophan and tyrosine derived from monooxygenases
Since these monooxygenases use dioxygen, you might expect that the enzymes would use the motifs described in the previous section to facilitate its reaction with dioxygen. In fact, the enzyme contains a metal ion (Fe2+) bound to a heme in the protein. In addition, the reduction products of dioxygen that are eventually used to hydroxylate the substrate stay bound to the enzyme.
Tyrosine 3-monooxygenase (Tyrosine hydroxylase)
Tyrosine hydroxylase is a homotetramer which uses an Fe ion and biopterin cofactor for hydroxylation. In the central nervous system is used in the biosynthesis of dihydroxyphenylalanine (DOPA), which is the rate limiting step in the catecholamine synthesis.
A possible mechanism for the rat enzyme is shown in Figure \(\PageIndex{11}\).
Figure \(\PageIndex{11}\): A possible mechanism for rat tyrosine hydroxylase, a monooxygenase. https://www.ebi.ac.uk/thornton-srv/m-csa/entry/134/
The Fe2+ ion binds O2. Some mechanisms show a single electron transfer to the bound O2 from the pterin ring to form Fe2+-O2-(bound superoxide) and a radical cation pterin. This is unstable and forms Fe2+-μ-peroxypterin, followed by heterolytic cleaves of the peroxo O-O bond. Ultimately, hdroxpternin and an Fe4+O oxospecies form which then hydroxylates tyrosine.
Figure \(\PageIndex{12}\) below shows an interactive iCn3D model of rat tyrosine hydroxylase with bound cofactor analogue and iron (2TOH).
Figure \(\PageIndex{12}\): Rat tyrosine hydroxylase with bound dihydrobiopterin analogue and iron (2TOH). (Copyright; author via source). Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...KeKiLDfmeNFfr7
Four monomers in the homotetramer are shown. The cofactor analog (HBI), 7,8-dihydrobiopterin, is shown in spacefill. Phenylalanine 300 has been self-hydroxylated by the enzyme to produce meta-tyrosine (MTY300), which along with the other key amino acids in the active site are shown labeled in CPK-colored sticks in the gray monomer. The pterin ring interacts through pi stacking with Phe 300, which facilitates self-hydroxylation. The Fe ion is far (5.6 Å) from the carbon on pterin which gets phosphorylated. This suggests that O2 might bridge the reacting pterin C- and the Fe ion in a Fe2+-μ-peroxypterin complex.
Tryptophan hydroxylase
This enzyme also uses a tetrahyropterin cofactor and Fe ion in the formation of he hydroxylating intermediates As in the case of tyrosine hydroxlase, both the amino acid substrate and the cofactor must be present for oxygen to be activated. Other, it only oxidizes Fe2+ to Fe3+. This serves as a protective mechanism so that O2 is not activated unnecessarily, which avoids the formation of soluble ROS.
Figure \(\PageIndex{13}\) shows a possible mechanism without catalytic residues for tryptophan hydroxylase
Figure \(\PageIndex{13}\): possible mechanism without catalytic residues for tryptophan hydroxylase adapted from Kenneth M. Roberts, Paul F. Fitzpatrick, https://doi.org/10.1002/iub.1144
Cytochrome P450s
The cytochrome P450 (CYP)consists of a large group of monooxygenases that contain a heme that absorbs maximally at 450 nm. They catalyze the following reaction:
RH + NAD(P)H + H+ + O2 → ROH + NAD(P)+ + H2O
An example includes cytochrome P450cam that hydroxylates camphor, a large aromatic completely nonpolar molecule. The enzyme has been called the"biological equivalent of a blowtorch" as it can, at room temperature, stereospecific hydroxylate nonactivated hydrocarbons at physiological temperature. This reaction proceeds without stereospecificity only at high temperatures in the absence of a catalyst. Remember from the previous chapter section that dioxygen is a ground state triplet radical which can't react well with carbons atoms (which are singlets) unless converted to a singlet state, a process which requires significant energy. Cytochrome P450s get around this problem by binding to an Fe ion in the heme to form common intermediates such as oxos, oxides, and peroxides. The molecular orbitals of the bound dioxygen are "singlet-like".
Remember from introductory chemistry that transition metal ligands are names with a specific nomenclature. Some are shown in Figure \(\PageIndex{14}\).
Figure \(\PageIndex{14}\): Common oxygen transition metal ligands
In naming η is the hapticity (the number of atoms of a ligand attached to a metal) and µ is the number of metal atoms bridged by a ligand. For Fe ions and oxygen ligands, some examples include FeIII–O2− (superoxo) and FeIV=O (ferryl-oxo)
If the aromatic substrate get oxidized, something must get reduced. That something is of course O2. Electrons for the reduction of O2 come from the oxidized substrate but also by injection of electrons through NAD(P)H. Cytochrome P450 are microsomal protein and most require another protein, NADPH-cytochrome P450 reductase (CPR). Before we look at the structure and mechanism of cytochrome P450, lets looks at this microsomal protein first. CPR catalyzes this reaction:
NADPH + oxidized CytoP450 (Fe3+-heme b) + H+ ↔ NADP+ + Oxidized CytoP450 (Fe2+-heme b)
The protein contains multiple domains and resulted from a fusion of gene from flavodoxin, which binds FMN) and a FAD reductases. In addition is contains an NADP binding domain. It ultimately accepts a hydride ion (2 electrons) from NADPH and then transfers electrons to FMN (in one electron steps). These are used to reduce the heme which then activates dioxygen (oxidation number of 0) for hydroxylation reactions by cytochrome P450. In the hydroxylated organic product and in water, oxygen has an oxidation number of -2.
Figure \(\PageIndex{15}\) below shows an interactive iCn3D model of rat NADPH-cytochrome P450 reductase (1AMO).
Figure \(\PageIndex{15}\): Rat NADPH-cytochrome P450 reductase (1AMO). (Copyright; author via source). Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...nMh9ByRbsWk4B8
NADP+ is labeled as NAP. The enzyme is quite unique in that it has binding sites for both FAD and FMN (similar to nitric-oxide synthase). The flavins are aligned for electron transfer. The cleft in the enzyme presumably binds cytochrome P450. The protein has short (20 amino acid) lumenal sections, followed by 20 amino acid membrane-spanning helix (not shown in the above structure) followed a large cytosolic domain, where it can interact with cytochrome P450, which is found in multiple places including the ER, microsomal, and mitochondrial membranes as well as the cytoplasm..
The cytochromes P450s containing a heme, which instead of reversibly carrying dioxygen (as in myoglobin and hemoglobin), activates dioxygen for hydroxylation reactions involving aromatic, nonpolar substrates. Hydroxylation of these substrates increases their solubility which facilitates their elimination from the body. Epoxide intermediates or products are often produced, which can open up through nucleophilic attack using an alcohol (sugar derivative), as illustrated in Figure \(\PageIndex{16}\).
Figure \(\PageIndex{16}\): Cytochrome P450 hydroxylation reaction to increase target solubility
This type of reaction also converts steroids to different biologically active one.
The hydroxylation reaction and can also lead to reactions with amines, including those on nucleotide bases in DNA, resulting in the formation of large adducts, as illustrated in Figure \(\PageIndex{17}\).
Figure \(\PageIndex{17}\): Cytochrome P450-mediated formation of carcinogens.
Hence cytochrome P450 can actually activate aromatic substrates to become carcinogens.
The cytochrome P450s family of genes/proteins are inducible on exposure to nonpolar aromatic molecules such as dioxin. These nonpolar molecules can enter the cytoplasm where they bind to the arylhydrocarbon receptor (AhR) which is bound to a heat shock protein, Hsp90. Upon binding of dioxin, TCDD, for example, the AhR.TCCD complex dissociates from Hsp90, and migrates to the nucleus where it binds a protein called Amt. The AhR-Amt complex serves as an enhancer/transcription factor, facilitating the transcription of the cytochrome P450 genes.
Figure \(\PageIndex{18}\) illustrates the activation of cytochrome P450 gene expression on exposure to nonpolar aromatic molecules such as dioxin.
Figure \(\PageIndex{18}\): Activation of cytochrome P450 and other gene expression on exposure to nonpolar aromatic molecules such as dioxin
On binding to a ligand, the AhR is activated and enters the nucleus, where it binds to ARNT on the aryl hydrocarbon response element (AhRE) and promotes transcription of downstream genes including cytochrome P450 family 1 subfamily A member 1 (CYP1A1) and interleukin-1 (IL-1). CYP1A1 is in the CYP1A family that promotes activation of procarcinogens a well as hydroxylation of steroid hormones like estrogens. It also participates in the metabolism of steroidal hormones including estrogens. ARNT is the aryl hydrocarbon receptor nuclear translocator, AhRE the aryl hydrocarbon response element; CYP1A1, XAP2 is the aryl hydrocarbon receptor interacting protein, AHRR is the aryl-hydrocarbon receptor repressor, IL-11 is interleukin 17, Hsp90 is heat shock protein 90 and p23 is prostaglandin E synthase 3
Dioxin has been shown to affect estrogen-mediated activities. Estrogens, small hydrophobic hormones derived from cholesterol, enter cell and bind to cytoplasmic estrogen receptors, which then dimerize and bind to the estrogen response element (ERE), initiating transcription. Tamoxifen, a drug derived from the yew plant, blocks the biological effects of the estrogen receptor. Although it binds to the estrogen receptor, it doesn't elicit the same conformational changes in the protein, which prevents the bound receptor from binding to the estrogen response element and recruiting other proteins needed for estrogen-dependent gene transcription. It is used in chemotherapy and prevention of estrogen-dependent breast cancer cells.
How does dioxin interfere with estrogen signaling? Ahr and ARnt contain a basic helix-loop-helix motif which mediate their interaction with DNA. Upon binding of the complex, detoxification genes are activated. The dioxin-Ahr-Arnt complex can also bind to the estrogen receptor, which can then lead to activation of genes containing an estrogen response element (ERE) in the absence of estrogen. However, if estrogen is present, inhibition of gene expression from ERE is observed. Dioxins can be potent dysregulators of estrogen-induced gene expression. Such changes in estrogen activity could help to explain the pro- and inhibitory effects of dioxin on estrogen-mediated cellular responses and possible effects of dioxin on the immune system and on cancer development.
Given the importance of the cytochrome P450s, we'll offer two variant portrayals of their mechanism. Figure \(\PageIndex{19}\) shows the overall catalytic cycle of the enzyme with associated redox changes in the generic substrate, RH, and the Fe heme ion.
Figure \(\PageIndex{19}\): Catalytic cycle of cytochrome P450 with the generic substrate RH and the Fe heme ion
The key hydroxylating agent appears to be formal FeO3+ shown in step 7.
A possible mechanism for the hydroxylation of estrone by cytochrome P450 from Bacillus megaterium is shown below in Figure \(\PageIndex{20}\). It follows the general catalytic cycle shown above.
Estrone + NADH + H+ + O2 → 2-hydroxyestrone + NAD+ + H2O
Figure \(\PageIndex{20}\): Possible mechanism for the hydroxylation of estrone by cytochrome P450 from Bacillus megaterium after https://www.ebi.ac.uk/thornton-srv/m...csa/entry/699/
As with the previous generic mechanism, the two added electrons derive from NADH which passed a hydride to FAD which passes single electrons on to FMN which them passes them onto the heme. The exact species for several parts of the mechanism are not completely clear. For instance, a Fe4+-oxo complex has been proposed.
Let's shown the structure of a human cytochrome P450 1A1 that is active in drug metabolism and also in the activation of benzo[a]pyrene, a component of cigarette smoke, into a carcinogen. Figure \(\PageIndex{21}\) below shows an interactive iCn3D model of Human Cytochrome P450 1A1 in complex with alpha-naphthoflavone (4I8V).
Figure \(\PageIndex{21}\): Human Cytochrome P450 1A1 in complex with alpha-naphthoflavone (4I8V). (Copyright; author via source). Click the image for a popup or use this external link: https://structure.ncbi.nlm.nih.gov/i...JGtJeHG1qswN59
The shows in sticks and labeled the active site heme and an inhibitor, α-naphthoflavone (labeled BHF) bound and enclosed in the active site. It's 2-phenyl group points toward the heme Fe ion.
Dioxygenases
An example of a dioxygenase is the cyclooxygenase activity of prostaglandin synthase. This enzyme, often just called cyclooxygenase or COX, is an integral membrane protein found in the ER membrane, and is a homodimer (with two hemes). It catalyzes two different reactions. One is the addition of two dioxygens to arachidonic acid - 20:4Δ5, 8, 11, 15 (which is liberated from the C2 position of phospholipid membranes by phospholipase A2 upon appropriate signaling) to form prostaglandin PGG2. This molecule, with 5 chiral centers, arises from arachidonic acid, which has one. The cyclooxygenase activity is buried in the membrane, from where the arachidonic acid can readily access its active site. The active site is at the end of a hydrophobic channel (arachidonic acid binding site) and stretches from the membrane-binding region to a buried heme. PGG2 can be further metabolized to PGH2 by the addition of two electrons to PGG2 by the hydroperoxidase activity of the enzyme, located at the other end of the enzyme. This activity forms an alcohol from the peroxide functional group in PGG2. There is one heme per monomer, which acts in both the cyclooxygenase and peroxidase activities. Each monomer of the dimer has both enzymatic activities. A possible abbreviated reaction mechanism is shown in Figure \(\PageIndex{22}\).
Figure \(\PageIndex{22}\): A possible abbreviated reaction mechanism for dioxygenase cyclooxygenase.
In summary:
- The carboxylate of arachidonic acid is coordinated to Arg 120 and Tyr 355.
- The C13 pro(S) H atom of arachidonic acid is close to Tyr 385 which allows its abstraction.
- This results in a radical centered on C11 that reacts with dioxygen to form a peroxyl radical
- Attack by dioxygen at C11 occurs from the side of the substrate opposite to that of hydrogen abstraction
- The oxygen radical at C11 cyclizes by attacking C9.
The C13 proS hydrogen atom (not proton) is remove from bound arachidonic acid by a free radical form of Tyr 385, which acts as an oxidizing agent. A site-specific mutants in which Tyr 385 is replaced by Phe is inactive. How is the Tyr free radical formed? Based on single electron standard reduction potential (0.9 V for Tyr and -0.2 to + 0.2 V for Fe3+ in the bound heme), it appears that the heme iron is not a potent enough oxidizing agent to accomplish this task. However, oxygen bound to the heme iron could be converted to a peroxide and form an Fe4+-oxo complex (which has also been proposed for cytochrome P450). The Fe4+ ion is a more potent oxidizing agent (standard reduction potential of approximately 1 V, sufficient for oxidation of Tyr 385. Another possibility is that the peroxide activator (in the formation of the ferryl-oxo ligand) is NO (nitric oxide, a free radical). NO is formed by immune cells (like macrophages) on immune activation. The NO might react with superoxide (also a radical, possibly formed during an oxidative burst in macrophages during immune stimulation) to form peroxynitrite (NO3-). This can donate an oxo group to the Fe3+ to form the Fe4+-oxo complex, which could then oxidize Tyr to the free radical form. There might be other mechanisms as well to generate the Tyr free radical, since just adding organic peroxides to the enzyme will generate it.. After abstraction of the proS H atoms, a carbon-centered free radical at C11 results which reacts with oxygen as shown below. The exact form of oxygen that reacts is unclear, but presumably is either a peroxy or activated singlet form.
Oxidases
This class of enzymes does not incorporate dioxygen into an organic substrate. Rather it accepts electrons released from an organic substrate, through intermediate electron carriers (such as ubiquinone and cytochrome C) to form superoxide (as in NADPH-oxidase), hydrogen peroxide (as in xanthine oxidase) or water (as in cytochrome C oxidase). The mechanism of cytochrome C oxidase again supports our expectations about enzymes that use dioxygen. Dioxygen binds metals in the enzyme. One oxygen atom binds a heme Fe2+ of cytochrome a3 which is bound to the enzyme, while the other binds a Cu1+ of Cu B. All oxygen reduction intermediates remain bound to the enzyme. Four electrons are added from four different cytochrome C molecules, which serve as mobile carriers of electrons.
We will explore cytochrome C oxidase in great detail in Chapter 19.1: Electron-Transfer Reactions in Mitochondria. Figure \(\PageIndex{23}\) shows cartoon versions of several oxidases.
Figure \(\PageIndex{23}\): Examples of oxidases
Another example of an oxidase is monoamine oxidase. Mitochondrial monoamine oxidase catalyzes the oxidative deamination of certain neurotransmitters after they have been taken up by post-synaptic neurons, in a process of inactivation. The reaction is shown in Figure \(\PageIndex{24}\).
Figure \(\PageIndex{24}\): Monoamine oxidase reaction
A Schiff base is formed which is then hydrolyzed, incorporating unlabeled oxygen into the oxidized molecule.
Biological Oxidations: Methane to CO2
In the previous chapter section, we discussed the progressive oxidation of methane by 2 electron loses to form methanol, formaldehyde, formic acid and CO2, with a progressive increase in oxidation number for the C by +2 (from -4 in methane to +4 in CO2), as reviewed in Figure \(\PageIndex{25}\).
Figure \(\PageIndex{25}\):: Progressive stages in the oxidation of methane
Methanotrophs are aerobic bacteria that use methane as a source of energy, converting it in a series of two electron oxidations as shown above, to carbon dioxide. The enzymes involved in this sequential process are methane monooxygenase, methanol dehydrogenase, formaldehyde dehydrogenase, and formate dehydrogenase. Methane monooxygenase exists in a soluble and membrane form, both of which are part of a larger complex. Both have a hydroxylase (which uses dioxygen to add O to methane) and the membrane form has recently been shown to be associated with have methanol dehydrogenase in a larger complex consisting of trimers of each enzyme (the hydroxylase and the dehydrogenase).
Heme Proteins
So far in this course, we have examined three different kinds of heme proteins..
- The first, hemoglobin (and myoglobin) serve as carriers of dioxygen. Even though they bind one of the best oxidizing agents around (dioxygen), the heme Fe2+ does not get oxidized to Fe3+. If it does, as in the case of met-Hb, the protein looses it ability to carry oxygen.
- Cytochrome C, on the other hand, does not bind dioxygen but rather serves as a carrier of electrons which get passed to dioxygen in Cytochrome C oxidase. Its Fe ion readily cycles between the 2+ and 3+ states as it serves as an electron carrier.
- Finally, the Fe2+ in the heme of the cytochrome P450s (so named since they have an absorbance maximum at 450 nm when they bind CO) does both. It binds dioxygen and cycles between the 2+ and 3+ states as it activates dioxygen for hydroxylation reactions.
The structure of the heme and amino acid ligands, along with their absorbance spectra, are shown in Table \(\PageIndex{3}\) below.
| hemoglobin | 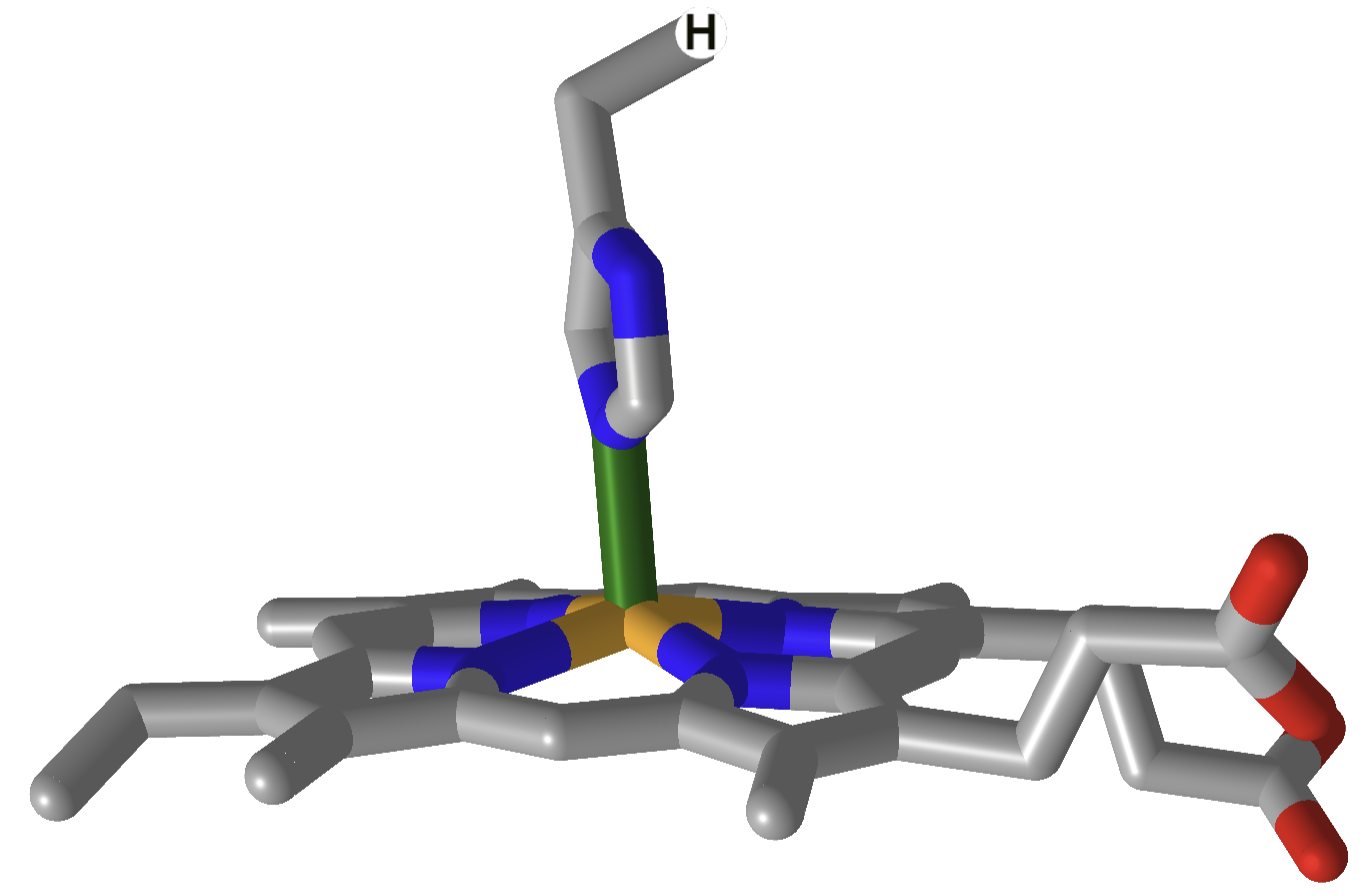 |
|
| cytochrome C | 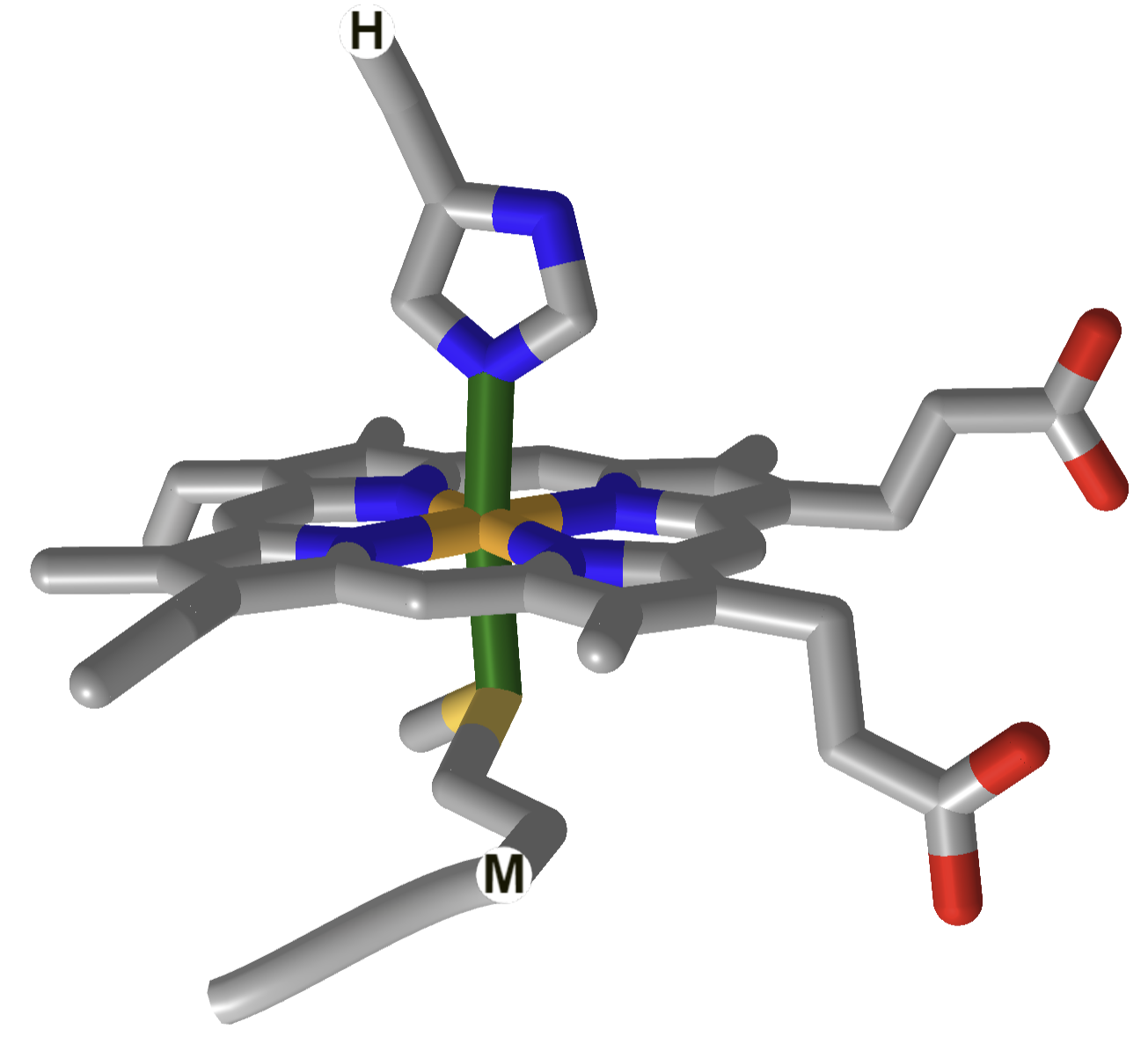 |
|
| Cytochrome P450 | 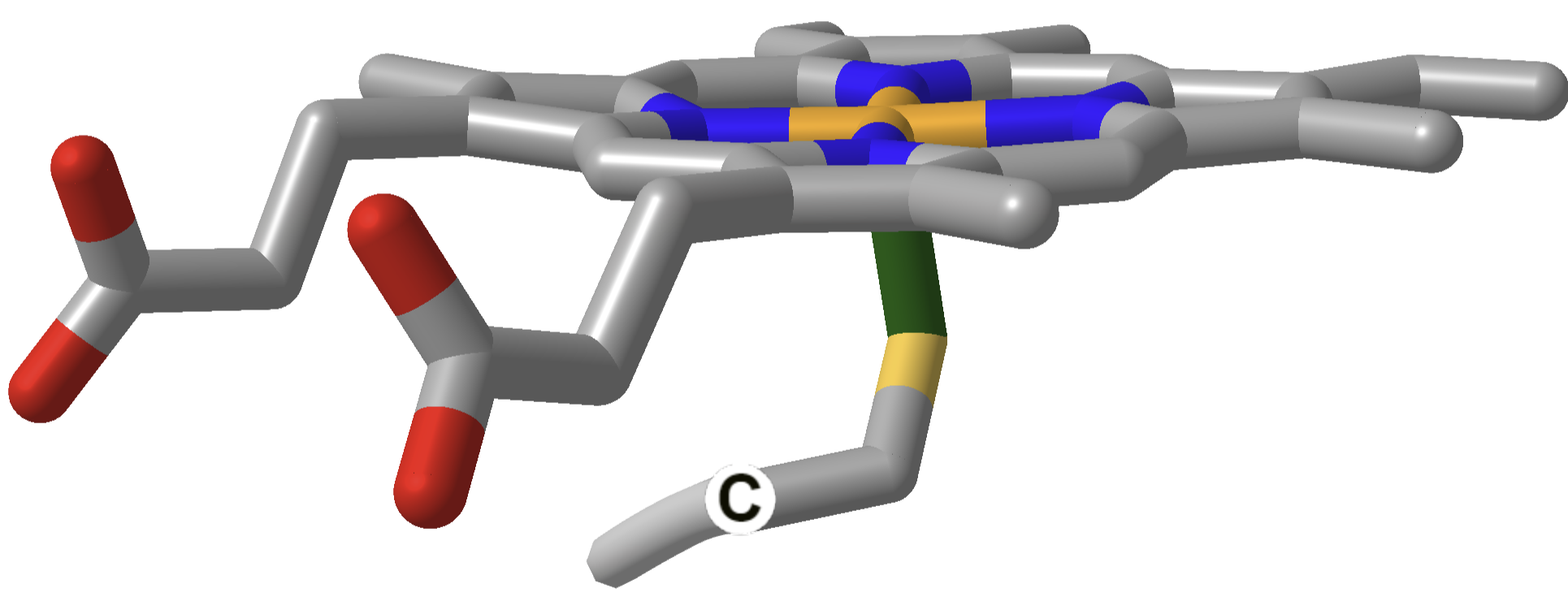 |
P450: Fujishiro et al. J Biol Chem. 2011 Aug 26; 286(34): 29941–29950. Published online 2011 Jun 30. doi: 10.1074/jbc.M111.245225. CC BY license.
Cyto C: Hulko et al. December 2011. Sensors 11(6):5968-80. DOI:10.3390/s110605968. Creative Commons Attribution 3.0 Unported
Hemoglobin: Nitzan et al. July 2014, Medical Devices: Evidence and Research 7(1):231-9. DOI:10.2147/MDER.S47319. Creative Commons Attribution-NonCommercial 3.0 Unported
How could heme serve such diverse functions? We can explain this by referring to one of the main themes of the course - structure mediates function. The environment of each heme must be different. Clearly the protein ligands coordinating the Fe ions are different. The 5th ligand is the proximal His in hemoglobin while dioxygen binds to the 6th site. In cytochrome C, the 5th and 6th ligands are His and Met, respectively. In cytochrome P450, the 5th site is occupied by Cys, and the 6th by dioxygen. Presumably the environments surrounding the hemes are different as well. Once again, we have seen analogous example in which chemical properties are influenced by the microenvironment. The pKa of a given amino acid side chain can vary considerably depending on the polarity of the local environment. Likewise, the standard reduction potential of tightly bound FAD/FADH2 depends on the microenvironment.
As we have seen (from the study of heme proteins and the oxidative enzymes of cells), transition metals such as Fe, Zn, and Cu have vital biological roles as binding sites and cofactors in many reactions. Yet they also pose problems since they can lead to oxidative damage in cells. As we saw with cytoplasmic metallothioneins, which bind to heavy metals and protect the cell from such damage, many proteins are involved in binding and regulation of transition metals in the cell. Integral membrane proteins are required to bind and transport these cations into the cytoplasm. Other proteins act as sensors of transition ion concentration (such as latent transcription factors which bind heavy metals and become active transcription factors for metallothioneins. Others act as chaperone proteins which bind metal ions and transfers them to apometalloproteins. Recent work has suggested transporters and chaperones involved in metal ion biology bind these ions with unusual coordination geometry, which presumably facilitates transfer of the ion to the apo-target protein.
The transition metals Zn and Fe are often found in E. Coli at a concentration of 0.1 mM, compared to Cu and Mn which are present at concentrations from 10 to 100 μM. Also, about one third of all proteins demonstrate specific binding of metal ions and can be classified as metalloproteins. Mass balance suggests that metal ions would be distributed in proteins with low, intermediate, and high metal binding affinity as well as in free pools, which which potentially be toxic to cells. Metalloproteins, depending on their Kd for metal ion binding, would hence be in various state of ligation. The free concentration of some ions (Cu and Zn) is so low that newly synthesized apoproteins which bind these ions would not obtain the ion from the free pool. In such cases, metal chaperones would be required.




.png?revision=1&size=bestfit&width=618&height=330)
.png?revision=1&size=bestfit&width=455&height=370)
.png?revision=1&size=bestfit&width=351&height=302)