BLUF Proteins
- Page ID
- 1587
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Introduction:
Living organisms need to be able to adapt and respond appropriately to the conditions around them. Therefore, the ability to adapt to environment changes such as the sunlight is crucial even for non-photosynethetic organisms. Higher plants (plants that contain a vascular system) have at least five types of sensory photoreceptors that increase the energy gained from photosynthesis. There are many different types of photoreceptors that use different chromophores that are receptive to different wavelengths of light. There are four families of blue-light photoreceptors with flavin chromophores: photoactive yellow protein (PYP), light-oxygen-voltage (LOV), cryptochromes and Blue Light Using Flavin (BLUF) proteins. The BLUF proteins are unique to the photorecepor family because they can show photo-induced proton-coupled electron transfer. On this page, we will focus on the BLUF proteins.
BLUF Protein:
The Blue Light using Flavin or BLUF protein was first discovered in 2002 in the unicellular flagellate Euglena gracilis (Iseki et al. 2002) and Rhodobacter sphaeroides.Rhodobacter sphaeroides is one of the most studied photosynthetic organism because of its structural and functional light reactions. The Rhodobacter sphaeroides, the BLUF domain is a blue light photo receptor involved in repressing the photosynthesis genes at the N-terminal region of the AppA protein. The AppA protein is required for increased photosystem gene expression upon transition of the facultatively photoheterotrophic bacterium Rhodobacter sphaeroides from aerobic to anaerobic photosynthetic conditions.
Euglena gracilis are photophobic and depend on a photo-activated adenyl cyclase (PAC) located at the base of the flagellum which increases the level of cAMP(messenger important in many biological proceses) when illuminated (Iseki et al. 2002). In other words, here the BLUF domain of PAC complexes serves as a blue light receptor in photophobic responses.
The BLUF domain is known to exist in many bacteria, including cyanobacteria. Due to genome sequencing, BLUF proteins can be found in both prokaryotes and eukaryotes which has lead to a variety of different organisms (Losi and Gartner 2008).
Structure:
The BLUF domain is a decamer. It ha a molecular weight of approximately 160kDa[1]. There are ten monomers. in each asymmetric unit.
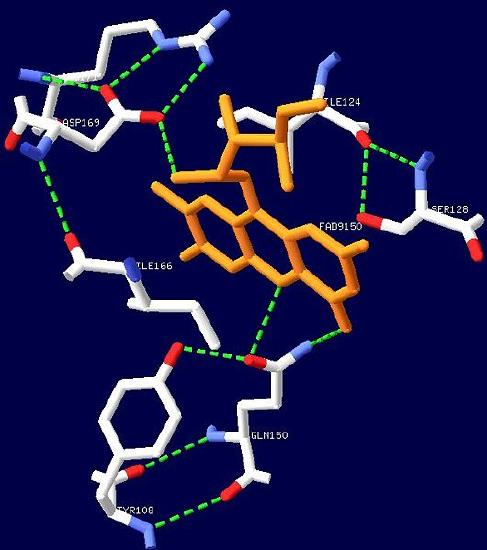
the blue light photoreceptor involved in the repression of photosynthesis genes in AppA protein.
ThThe BLUF domain is known to exist in many bacteria, including cyanobacteria
They were introduced as "light-triggered switches that control enzyme activity or gene expression in response to blue light, remaining activated for seconds or even minutes after stimulation." Euglena gracilis are photophobic and depend on a photo-activated adenyl cyclase (PAC) located at the base of the flagellum which increases the level of cAMP(messenger important in many biological proceses) when illuminated (Iseki et al. 2002). Rhodobacter sphaeroides controls gene expression realtin to photosynthesis through AppA (a light-sensitive protein) which interacts with protein called PpsR (a DNA-binding protein) (Masuda and Bauer 2002). Yet, AppA and PAC are two of the many examples of the photo-sensitive proteins carrying the BLUF domain(Gomelsky and Klug 2002).
Due to genome sequencing, BLUF proteins can be found in both prokaryotes and eukaryotes which has lead to a variety of different organisms (Losi and Gartner 2008).
References:
- Gomelsky M, Klug G. BLUF: a novel FAD-binding domain involved in sensory transduction in microorganisms. Trends Biochem Sci. 2002;27:497–500. doi: 10.1016/S0968-0004(02)02181-3. [PubMed] [Cross Ref]
- Losi A, Gartner W. Bacterial bilin- and flavin-binding photoreceptors. Photochem Photobiol Sci. 2008;7:1168–1178. doi: 10.1039/b802472c. [PubMed] [Cross Ref]
- Masuda S, Bauer CE. AppA is a blue light photoreceptor that antirepresses photosynthesis gene expression in Rhodobacter sphaeroides. Cell. 2002;110:613–623. doi: 10.1016/S0092-8674(02)00876-0. [PubMed] [Cross Ref]
Original..
In molecular biology, the BLUF domain (sensors of blue-light using FAD) is a FAD-binding protein domain. They are present in various proteins, primarily from bacteria, for example a BLUF domain is found at the N-terminus of the AppA protein from Rhodobacter sphaeroides. The BLUF domain is involved in sensing blue-light (and possibly redox) using FAD and is similar to the flavin-binding PAS domains and cryptochromes. The predicted secondary structure reveals that the BLUF domain has a novel FAD-binding fold.
Under construction... please check back later

