43: API-20E multitest strip
- Page ID
- 3692
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- Learn how to perform and interpret the miniaturized, multi-test technique for bacterial identification
API - 20E TEST STRIP
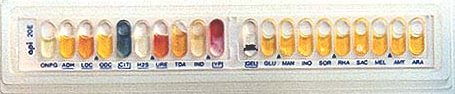
This API-20E test strip (from bioMerieux, Inc.) is used to identify the enteric gram negative rods (although API makes a variety of other test strips for yeast, Staph, anaerobes, etc.) 20 separate test compartments are on the strip, all dehydrated. A bacterial suspension is used to rehydrate each of the wells. Some of the wells will have color changes due to pH differences: others produce end products that have to be identified with reagents. A profile number is determined from the sequence of + and test results, then looked up in a code book having a correlation between numbers and bacterial species.
MATERIALS NEEDED
- agar plates of bacterial species
- 0.85% NaCl solutions (5 ml)
- sterile Pasteur pipettes + bulbs
- sterile mineral oil
- API 20E test strip (for oxidase - gram negative rods)
- API test strip incubation chamber AFTER INCUBATION: 10% FeCl3, Barrett's reagents
- A and B, Kovac's reagent
THE PROCEDURES
Prepare a suspension of the bacteria in the saline tube
- Inoculate a large colony (2-3 mm diameter) of the bacterium (a young pure culture) into the 0.85% NaCl solution.
- Make sure that the suspension is homogenous and without clumps of floating bacteria.
Inoculate the API strip
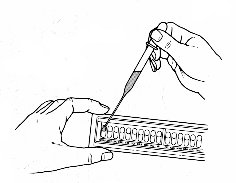
- Holding the strip at a slight angle up from the table top, you will now inoculate the bacterial suspension into each well with the sterile pipette.
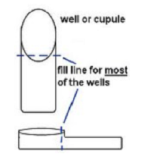
- Touch the end of the pipette to the side of the cupule, allowing capillary action to draw the fluid into the well as you slowly squeeze the bulb. This should eliminate any bubbles forming in the wells. Each well should be filled up to the neck (see diagram).
- CIT, VP, and GEL have boxes around their names. These test wells will be filled all the way up to the top of the well.
- LDC, ODC, ADH, H2S, and URE are filled as described in step B, but they will then be filled up to the top with sterile mineral oil.
Incubate the strip in its chamber
- The bottom of the incubation chamber has small indented wells in the bottom: fill it with water just enough to fill these indentations (about 5 ml).
- Place the strip into this bottom. There should not be so much water that it slops onto the API strip.
- Place the top of the incubation chamber over the bottom, and label it.
- Place the strip at 37º C for 18-24 hours.
INTERPRETATION
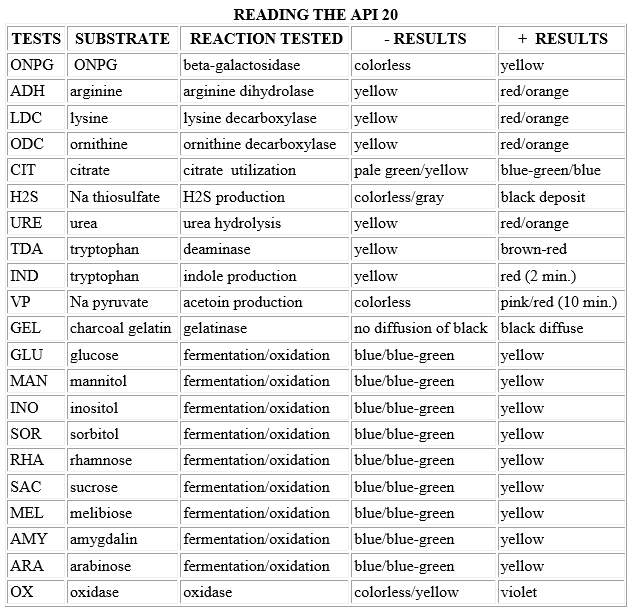
- Add the proper reagents to the compartments:
- 1 drop of Kovac's to the IND (read within a couple of minutes)
- 1 drop of Barritt's A and B to VP (a + reaction may take up to 10 minutes)
- 1 drop of FeCl3 to TDA
- Read all other tests as described (chart below) without reagents.
- Record results on the diagram handed out to you in lab (1, 2, or 4 points for + reaction, 0 points for - reaction). The oxidase test reaction should be negative, and is added as the last test result.
- Three test reactions are added together at a time to give a 7-digit number, which can then be looked up in the codebook.
Note
Additional tests such as motility, growth on MacConkey agar, nitrate reduction and O-F glucose can increase the chances of a good identification. Your instructor may have you run or may not. The basic tests are really all that are absolutely necessary for an identification.
QUESTIONS
- What is the purpose of the water in the tray?
- What is the function of the mineral oil?
- What are the advantages of this test (compared to regular biochemical tube media)?
- What are the disadvantages of this test?
Contributors and Attributions
-
Jackie Reynolds, Professor of Biology (Richland College)