6.7: Cell Transport
- Page ID
- 92602
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Look at the big windows and glass doors in this house. Imagine all the light they must let in on a sunny day. Now imagine living in a house that has walls without any windows or doors. Nothing could enter or leave. Or imagine living in a house with holes in the walls instead of windows and doors. Things could enter or leave, but you couldn’t control what came in or went out. Only if a house has walls with windows and doors that can be opened or closed you can control what enters or leaves. For example, windows and doors allow you to let in light and the family dog and keep out rain and bugs.
.jpg?revision=1&size=bestfit&width=441&height=285)
Transport Across Membranes
If a cell were a house, the plasma membrane would be walls with windows and doors. Moving things in and out of the cell is an important role of the plasma membrane. It controls everything that enters and leaves the cell. There are two basic ways that substances can cross the plasma membrane: passive transport, which requires no energy; and active transport, which requires energy. Passive transport is explained in this section and Active transport is explained in the next section, Active Transport and Homeostasis. Various types of cell transport are summarized in the concept map in Figure \(\PageIndex{2}\).
Transport Without Energy
Passive transport occurs when substances cross the plasma membrane without any input of energy from the cell. No energy is needed because the substances are moving from an area where they have a higher concentration to an area where they have a lower concentration. Water solutions are very important in biology. When water is mixed with other molecules this mixture is called a solution. Water is the solvent and the dissolved substance is the solute. A solution is characterized by the solute. For example, water and sugar would be characterized as a sugar solution. More the particles of a solute in a given volume, the higher the concentration. The particles of solute always move from an area where it is more concentrated to an area where it is less concentrated. It’s a little like a ball rolling down a hill. It goes by itself without any input of extra energy.
The different categories of cell transport are outlined in Figure \(\PageIndex{2}\). Cell transport can be classified as follows:
- Passive Transport which includes
- Simple Diffusion
- Osmosis
- Facilitated Diffusion
- Active Transport can involve either a pump or a vesicle
- Pump Transport can be
- primary
- secondary
- Vesicle Transport can involve
- Exocytosis
- Endocytosis which includes
- Pinocytosis
- Phagocytosis
- Receptor-Mediated Endocytosis
- Pump Transport can be
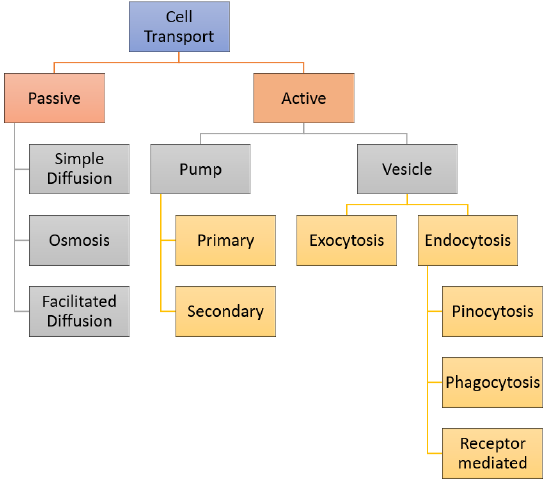
Figure \(\PageIndex{2}\): The Cell Transport Concept Map illustrates various types of cell transports that happen at the plasma membrane
Simple Diffusion
Diffusion Although you may not know what diffusion is, you have experienced the process. Can you remember walking into the front door of your home and smelling a pleasant aroma coming from the kitchen? It was the diffusion of particles from the kitchen to the front door of the house that allowed you to detect the odors. Diffusion is defined as the net movement of particles from an area of greater concentration to an area of lesser concentration.

The molecules in a gas, a liquid, or a solid are in constant motion due to their kinetic energy. Molecules are in constant movement and collide with each other. These collisions cause the molecules to move in random directions. Over time, however, more molecules will be propelled into the less concentrated area. Thus, the net movement of molecules is always from more tightly packed areas to less tightly packed areas. Many things can diffuse. Odors diffuse through the air, salt diffuses through water and nutrients diffuse from the blood to the body tissues. This spread of particles through the random motion from an area of high concentration to an area of lower concentration is known as diffusion. This unequal distribution of molecules is called a concentration gradient. Once the molecules become uniformly distributed, a dynamic equilibrium exists. The equilibrium is said to be dynamic because molecules continue to move, but despite this change, there is no net change in concentration over time. Both living and nonliving systems experience the process of diffusion. In living systems, diffusion is responsible for the movement of a large number of substances, such as gases and small uncharged molecules, into and out of cells.
Osmosis
Osmosis is a specific type of diffusion; it is the passage of water from a region of high water concentration through a semi-permeable membrane to a region of low water concentration. Water moves in or out of a cell until its concentration is the same on both sides of the plasma membrane.
Semi-permeable membranes are very thin layers of material that allow some things to pass through them but prevent other things from passing through. Cell membranes are an example of semi-permeable membranes. Cell membranes allow small molecules such as oxygen, water carbon dioxide, and oxygen to pass through but do not allow larger molecules like glucose, sucrose, proteins, and starch to enter the cell directly.
The classic example used to demonstrate osmosis and osmotic pressure is to immerse cells into sugar solutions of various concentrations. There are three possible relationships that cells can encounter when placed into a sugar solution. Figure \(\PageIndex{4}\) shows what happens in osmosis through the semi-permeable membrane of the cells.
- The concentration of solute in the solution can be greater than the concentration of solute in the cells. This cell is described as being in a hypertonic solution (hyper = greater than normal). The net flow or water will be out of the cell.
- The concentration of solute in the solution can be equal to the concentration of solute in cells. In this situation, the cell is in an isotonic solution (iso = equal or the same as normal). The amount of water entering the cell is the same as the amount leaving the cell.
- The concentration of solute in the solution can be less than the concentration of solute in the cells. This cell is in a hypotonic solution (hypo = less than normal). The net flow of water will be into the cell.
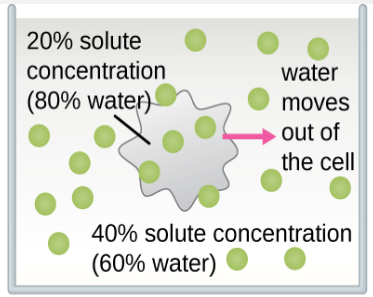
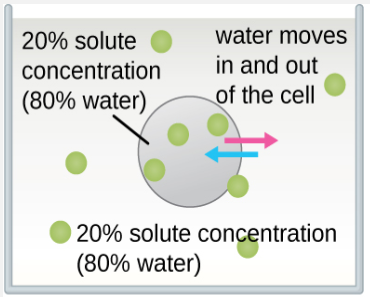
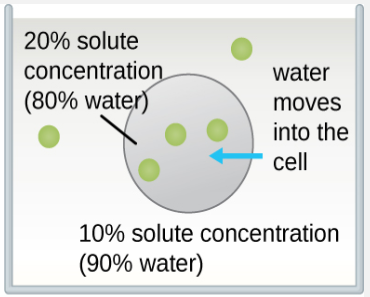
Figure \(\PageIndex{5}\) demonstrates the specific outcomes of osmosis in red blood cells.
- Hypertonic solution. The red blood cell will appear to shrink as the water flows out of the cell and into the surrounding environment.
- Isotonic solution. The red blood cell will retain its normal shape in this environment as the amount of water entering the cell is the same as the amount leaving the cell.
- Hypotonic solution. The red blood cell in this environment will become visibly swollen and potentially rupture as water rushes into the cell.
Facilitated Diffusion
Water and many other substances cannot simply diffuse across a membrane. Hydrophilic molecules, charged ions, and relatively large molecules such as glucose all need help with diffusion. The help comes from special proteins in the membrane known as transport proteins. Diffusion with the help of transport proteins is called facilitated diffusion. There are several types of transport proteins, including channel proteins and carrier proteins (Figure \(\PageIndex{6}\))
- Channel proteins form pores, or tiny holes, in the membrane. This allows water molecules and small ions to pass through the membrane without coming into contact with the hydrophobic tails of the lipid molecules in the interior of the membrane.
- Carrier proteins bind with specific ions or molecules, and in doing so, they change shape. As carrier proteins change shape, they carry the ions or molecules across the membrane.
Review
- What is the main difference between passive and active transport?
- Summarize three different ways that passive transport can occur, and give an example of a substance that is transported in each way.
- Explain how transport across the plasma membrane is related to the homeostasis of the cell.
- Why can generally only very small, hydrophobic molecules across the cell membrane by simple diffusion?
- Explain how facilitated diffusion assists in osmosis in cells. Be sure to define osmosis and facilitated diffusion in your answer.
- Imagine a hypothetical cell with a higher concentration of glucose inside the cell than outside. Answer the following questions about this cell, assuming all transport across the membrane is passive, not active.
- Can the glucose simply diffuse across the cell membrane? Why or why not?
- Assuming that there are glucose transport proteins in the cell membrane, which way would glucose flow – into or out of the cell? Explain your answer.
- If the concentration of glucose was equal inside and outside of the cell, do you think there would be a net flow of glucose across the cell membrane in one direction or the other? Explain your answer.
- What are the similarities and differences between channel proteins and carrier proteins?
- True or False. Only active transport, not passive transport, involves transport proteins.
- True or False. Oxygen and carbon dioxide can squeeze between the lipid molecules in the plasma membrane.
- True or False. Ions easily diffuse across the cell membrane by simple diffusion.
- Controlling what enters and leaves the cell is an important function of the:
- nucleus
- vesicle
- plasma membrane
- Golgi apparatus
Explore More
Check out this video to learn more about osmosis and tonicity:
Attributions
- House by Moyan Brenn from Italy, CC BY 2.0 via Wikimedia Commons
- Flowchart by Mandeep Grewal, CC BY-NC 3.0
- Simple diffusion by LadyofHats Mariana Ruiz Villarreal released into the public domain via Wikimedia Commons
- Tonicity by CNX OpenStax, CC BY 4.0 via Wikimedia Commons
- Osmotic pressure on blood cells by LadyofHats Mariana Ruiz Villarreal released into the public domain via Wikimedia Commons
- Facilitated diffusion by LadyofHats Mariana Ruiz Villarreal released into the public domain via Wikimedia Commons
- Text adapted from Human Biology by CK-12 licensed CC BY-NC 3.0


