9.4: Cancer risk is heritable
- Page ID
- 147425
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)One of the reasons to discuss cancer in a genetics course is that a person's risk for developing cancer is itself a heritable trait.
Cancer risk can be inherited in a Mendelian pattern
Null mutations in tumor supressors or gain-of-function mutations in protooncogenes can increase a person's risk of developing cancer so much that they become readily observable on a pedigree -- and of course the risk allele is inherited with a Mendelian pattern. Perhaps the prototypical example of Mendelian inheritance of cancer risk is the RB1 gene, which encodes for the tumor suppressor protein Rb. The Rb protein is involved in signaling to a cell to proliferate (divide). If both alleles of this gene are mutated in a retinal cell, the protein is inactivated and the cells grow uncontrollably, resulting in development of retinoblastoma cancer, hence the "RB" in the name 'pRb'. Thus most pRb knock-outs occur in retinal tissue when UV radiation-induced mutation inactivates all healthy copies of the gene, but pRb knock-out has also been documented in certain skin cancers in patients from New Zealand where the amount of UV radiation is significantly higher.
Researchers studying retinoblastoma noticed two patterns of cancer development: a bilateral (both eyes), familial (inherited) form and a unilateral (one eye), sporadic form. Patients in families where retinoblastoma was common were also over six times more likely to develop other types of cancer later in life, compared to individuals with sporadic retinoblastoma.[10] This highlighted the fact that mutated pRb could be inherited and lent support for the two-hit hypothesis: that only one working allele of a tumour suppressor gene is necessary for its function (the mutated gene is recessive), and so both need to be mutated before the cancer phenotype will appear. In the familial form, a mutated allele is inherited along with a normal allele. In this case, should a cell sustain only one mutation in the other RB gene, all pRb in that cell would be ineffective at inhibiting cell cycle progression, allowing cells to divide uncontrollably and eventually become cancerous. Furthermore, as one allele is already mutated in all other somatic cells, the future incidence of cancers in these individuals is observed with linear kinetics. The working allele need not undergo a mutation per se, as loss of heterozygosity (LOH) is frequently observed in such tumours.

The pedigree from a family with familial retinoblastoma. Wiggs et al, N Engl J Med 1988; 318:151-7.
However, in the sporadic form, both alleles would need to sustain a mutation before the cell can become cancerous. This explains why sufferers of sporadic retinoblastoma are not at increased risk of cancers later in life, as both alleles are functional in all their other cells. Future cancer incidence in sporadic pRb cases is observed with polynomial kinetics, not exactly quadratic as expected because the first mutation must arise through normal mechanisms, and then can be duplicated by LOH to result in a tumour progenitor.
Cancer risk is also a complex trait
While familial cancer risk following a Mendelian pattern of inheritance is useful for identifying protooncogenes and tumor suppressors, there are many genes with alleles that modestly increase or decrease a person's risk of cancer. (A 2016 study estimated the narrow-sense heritability of cancer overall as 0.33.) A person's lifestyle and environment -- whether they smoke, whether they live in a place with PFOAs in the water, whether they get regular exercise, whether they have ready access to nutrient-dense food -- also impacts a person's lifetime cancer risk.
Thus, cancer is a prototypical complex trait, and it is therefore not surprising that researchers have used genome-wide association studies (GWAS) to identify quantitative trait loci (QTLs). For example, a study of over 200,000 women identified 48 loci associated with the development of breast cancer. Some of these loci were already known to affect proliferation or colony formation, but many were not. Further functional characterization of these loci may hold future discoveries for the way that cells change as they become carcinogenic. It also reinforces the importance of telling physician if there's a history of cancer in your family, because these risk alleles are heritable just like alleles for overt (physically observable) phenotypes.
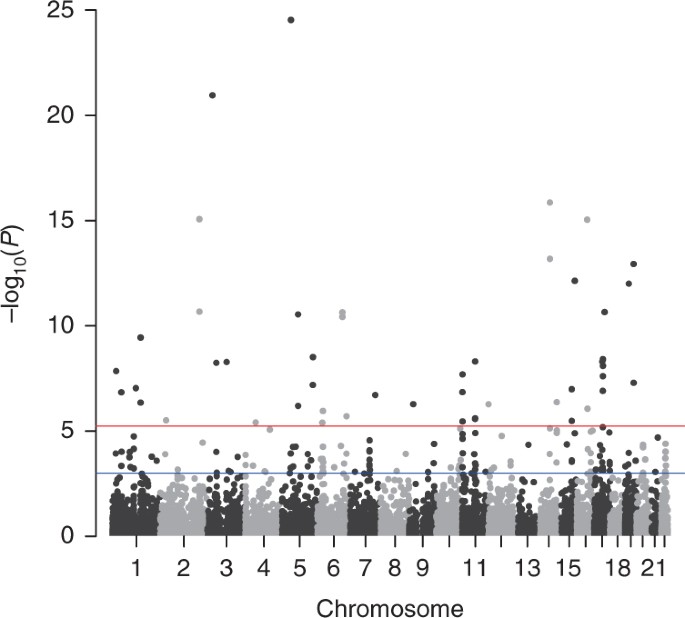
A Manhattan plot from a breast cancer genome-wide association study. From Wu et al, Nat Genet 50: 968-978 (2018)
Sources:
https://en.wikipedia.org/wiki/Retinoblastoma_protein
Mucci et al, JAMA 2016; 315(1):68-76
Wiggs et al, N Engl J Med 1988; 318:151-7.
Wu et al, Nat Genet 50: 968-978 (2018)

