13.2: Posttranscriptional Control of Gene Expression
- Page ID
- 88977
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Not too long ago, we thought that very little of the eukaryotic genome was ever transcribed. We also thought that the only noncoding RNAs were tRNAs and rRNAs. Now we know that other RNAs play roles in gene regulation and in the degradation of spent cellular DNA or unwanted foreign DNA. These are discussed in detail next.
13.2.1. Riboswitches
The riboswitch is a bacterial transcription mechanism for regulating gene expression. While this mechanism is not specifically posttranscriptional, it is included here because the action occurs after transcription initiation and aborts the completion of an mRNA. When the mRNA for an enzyme in the guanine synthesis pathway is transcribed, it folds into stem-and-loop structures. Enzyme synthesis will continue for as long as the cell needs to make guanine. But if guanine accumulates in the cell, excess guanine will bind stem-loop elements near the 5’ end of the mRNA, causing the RNA polymerase and the partially completed mRNA to dissociate from the DNA, prematurely ending transcription.
The ability to form folded stem-loop structures at the 5’ ends of bacterial mRNAs seems to have allowed the evolution of translation-regulation strategies. Guanine interaction with the stem-loop structure of an emerging 5′ mRNA can abort its own transcription; similarly, small metabolite/mRNA and even protein/mRNA interactions can also regulate (in this case prevent) translation. The basis of guanine riboswitch-regulation of the expression of a guanine synthesis pathway enzyme is shown below in Figure 13.1.

As we will see shortly, 5’ mRNA folded structures also play a role in eukaryotic translation regulation.
232 Riboswitches Interrupt Bacterial Transcription
233 Small Metabolites Also Regulate Bacterial mRNA Translation
13.2.2 CRISPR/Cas: A Prokaryotic Adaptive Immune System
In higher organisms, the immune system is adaptive. It remembers prior exposure to a pathogen and can thus mount a response to a second exposure to the same pathogen. The discovery of an “adaptive immune system” in many prokaryotes was therefore something of a surprise.
The CRISPR story begins in 1987 in Japan, with the discovery by Y. Ishino (et al.) of a cluster of regularly interspaced short palindromic DNA sequences in E. coli. Clustered DNA repeats were known, of course, but they were always consecutive and uninterrupted by other sequences (think rRNA genes). Ishino’s cluster was (at the time) of unknown origin and function. In 1993 similar clustered repeats were found in several strains of Mycobacterium tuberculosis (the cause of tuberculosis), but the interspaced sequences interrupting the palindromic repeats differed between the strains. By 2001 these clustered, interrupted repeats, by then found in many prokaryotes (both bacterial and archaebacterial), were named CRISPR (Clustered Regularly Interspaced Short Palindromic Repeats).
The discovery of an array of Cas-gene repeats was linked to some of the CRISPR repeats in 2002. This was followed in 2005 by the revelation that some of the interspaced sequences were derived from nonhost origins, specifically phage DNA and plasmids, and in the same year, the protein encoded by the Cas gene was shown to have helicase and nuclease activity. This led to a suggestion that CRISPR and Cas were part of a bacterial adaptive immune system, a bacterial response to phage infection or other invasive DNA and (for the lucky bacterial survivor), a protective mechanism against a second infection! In 2012 Jennifer Doudna and Emmanuelle Charpentier showed how the CRISPR/Cas9 system in streptococcal bacteria could rapidly and accurately edit any DNA sequence, including genomic DNA. Let’s look more closely at CRISPR and Cas DNAs to see how they work as an adaptive immune system and how they can be used to edit DNA.
CRISPR RNAs are derived from phage transcripts that have interacted with CRISPR associated (Cas) proteins. They make up the CRISPR/Cas system, which seems to have evolved to fight off viral infections by targeting phage DNA for destruction. When viral DNA gets into a cell during a phage infection, it can generate a CRISPR/Cas-gene array in the bacterial genome, with spacer DNA sequences separating repeats of the CRISPR genes. These remnants of a phage infection are the memory of this prokaryotic immune system. When a phage attempts to re-infect a previously exposed cell, spacer RNAs and Cas genes are transcribed. After Cas mRNA translation, the Cas protein and spacer RNAs will engage and target the incoming phage DNA for destruction to prevent infection. CRISPR/Cas systems (there are more than one!) remember prior phage attacks and transmit that memory to progeny cells. The CRISPR/Cas9 system in Streptococcus pyogenes is one of the simplest of the CRISPR/Cas immune defense systems (Figure 13.2).

The CRISPR/Cas gene array consists of the following components:
- Cas: Genes native to host cells
- CRISPR: Twenty-four to forty-eight base-pair repeats native to host cells
- Spacer DNA: DNA between CRISPR repeats—typically, phage DNA from prior phage infection or plasmid transformation
- Leader DNA: DNA containing a promoter for the transcription of CRISPR/spacer RNA
- tracr gene: Gene encoding a transcription-activator (tracr) RNA (but not included in all systems)
Let’s look at CRISPR/Cas in action.
13.2.2.a The CRISPR/Cas “Immune” Response
Consider the mechanism of action of this prokaryotic immune system. The action begins when infectious phage DNA gets into the cell (Figure 13.3).

Let’s summarize what has happened here:
- Incoming phage DNA was detected after phage infection (1).
- The tracr (2) and Cas9 (3) genes are transcribed, along with the CRISPR/phage-derived spacer region (4). Cas9 mRNAs are then translated to make the Cas9 protein. Remember, the spacer DNAs in the CRISPR region are the legacy of a prior phage infection.
- CRISPR/phage-derived spacer transcripts forms H-bonds with a complementary region of the tracr RNA (5) and the hybridized RNAs associate with Cas9 proteins (6).
- Cas9 endonucleases hydrolyze spacer RNA from CRISPR RNA sequences (7). The spacer RNAs remain associated with the complex, while the actual, imperfectly palindromic CRISPR sequences (shown in blue in the illustration) fall off.
In the next steps we’ll look at how phage-derived spacer DNA from a prior infection protect progeny bacteria from a new phage infection. When transcribed, these spacer RNAs are now called guide RNAs (gRNAs). They will guide mature Cas9/tracr-RNA/spacer RNA complexes to the new incoming phage DNA. Figure 13.4 illustrates these events and subsequent events.
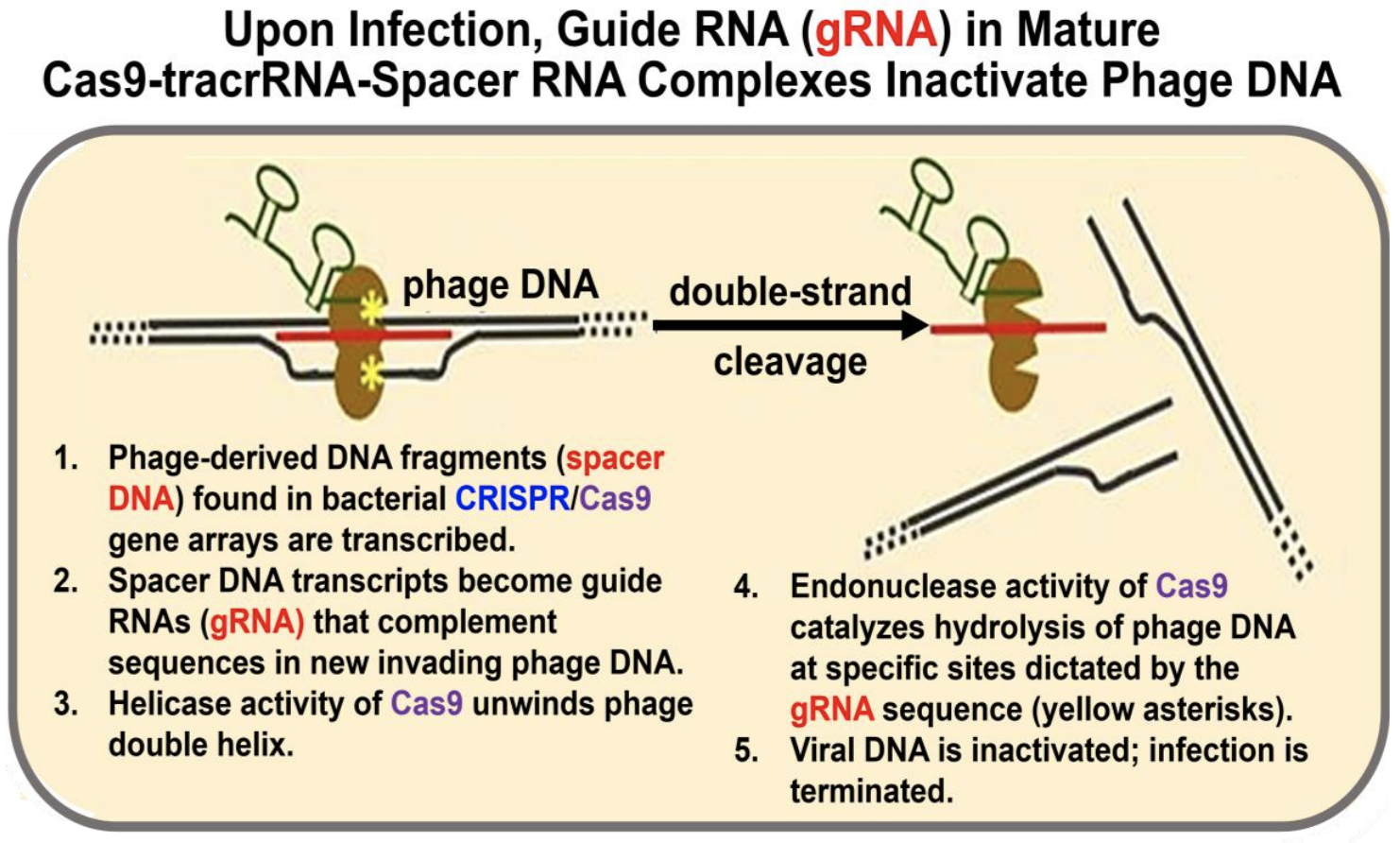
Once again, let’s summarize:
a) Spacer RNA (i.e., gRNA) in the complex targets incoming phage DNA.
b) Cas helicase unwinds the incoming phage DNA at complementary regions.
c) gRNA H-bonds to the incoming phage DNA.
d) Cas endonucleases create a double-stranded break (hydrolytic cleavage) at specific sites in the incoming phage DNA. Because precise cleavage is guided by RNA molecules, CRISPR/Cas endonucleases are classified as type V restriction enzymes.
e) The incoming phage DNA is destroyed, and a new phage infection is aborted.
Check out More About CRISPR to learn more about how bacteria acquire spacer DNAs (and therefore how this primitive adaptive immune system “remembers”) in the first place.
13.2.2.b Using CRISPR/Cas to Edit/Engineer Genes
Early studies demonstrated the reproducible cleavage of incoming phage DNA at specific nucleotides. Several labs quickly realized that it might be possible to adapt the system to cut DNA at any specific nucleotide in a target DNA!
It has turned out that the system works both in vivo and in vitro, allowing virtually unlimited potential for editing genes and RNAs in a test tube—or in any cell. Here is the basic process:
a) Engineer gDNA with a Cas-specific DNA sequence that targets a desired target in genomic DNA.
b) Fuse the gDNA to tracr DNA to make a single guide DNA (sgDNA), so that it can be made as a single guide transcript (sgRNA).
c) Engineer a CRISPR/Cas9 gene array that substitutes this sgDNA for its original spacer DNAs.
d) Place the engineered array into a plasmid next to regulated promoters.
e) Transform cells by “electroporation”—which works for almost any cell type!
f) Activate the promoter to transcribe the CRISPR/Cas9 genes….
For demonstrating the power of CRISPR-Cas precision gene editing, Jennifer Doudna and Emmanuelle Charpentier earned the 2020 Nobel Prize for Chemistry. The applications are indeed powerful… and controversial!
13.2.2.c CRISPR—The Power and the Controversy
The application of gene editing with CRISPR/Cas systems has already facilitated studies of gene function in vitro, in cells and in whole organisms. For a description of CRISPR/Cas applications already on the market see CRISPR Apps from NEB. The efficiency of specific gene editing using CRISPR/Cas systems holds great promise for understanding basic gene structure and function, for determining the genetic basis of disease, and for accelerating the search for gene therapies. Here are a few examples of how CRISPR/Cas editing is being applied.
- One can now engineer an sgRNA with mutations that target specific sites almost anywhere in chromosomal DNA. The sgRNA is cloned into the CRISPR/Cas9 array on a plasmid. After transformation of appropriate cells, the engineered CRISPR/Cas9 forms a complex with target-DNA sequences. After both strands of the target DNA have been nicked, DNA repair can insert the mutated guide sequences into the target DNA. The result is loss or acquisition of DNA sequences at specific, exact sites, or precision gene editing. The ability to do this in living cells has excited scientists in both the basic and clinical research communities.
- Before transforming cells, one can engineer the CRISPR/Cas9 gene array onto the plasmid to eliminate both endonuclease activities from the Cas protein. Upon transcription of the array in transformed cells, the CRISPR/Cas9-sgRNA still finds an sgRNA-targeted gene. However, lacking Cas protein endonuclease activities, the complex that forms just sits there blocking transcription. This technique is sometimes referred to as CRISPRi (CRISPR interference), by analogy to RNA interference or RNAi (see section 13.2.3.a below). Applied to organisms (and not just in vitro or to cells), it mimics the much more difficult knockout mutation experiments that have been used in studies of the behavior of cells or organisms that have been made unable to express a specific protein.
Several newer, highly efficient CRISPR/Cas systems are exciting prospects for rapid, targeted gene therapies to fight disease and their potential to alter entire populations using gene drive technologies (see Gene Drive) to insert modified genes into germline cells of a target organism. Gene drive could eliminate pesticide resistance in unwanted insects and herbicide resistance in undesirable plants. It could even erase whole mosquito or invasive species populations. For a recent proof-of-concept research, see Kryou et al. (2018) A CRISPR-Cas9 Gene Drive Targeting doublesex Causes Complete Population Suppression In Caged Anopheles gambiae Mosquitoes. Nat. Biotech. 1062-1066. But the use of CRISPR-based gene drive to control populations is also controversial (see J. Adler, (2016) A World Without Mosquitoes. Smithsonian, 47, 36-42).
- It is possible to delete an entire chromosome from cells. This bit of “global” genetic engineering depends on our ability to identify multiple unique sequences on a single chromosome and then to target these sites for CRISPR/Cas. When the system is activated, the chromosome is cut at those sites, fragmenting it beyond the capacity of DNA-repair mechanisms to fix the situation. Imagine the possibilities for therapeutically removing from a human genome, aneuploidies—for example, extra chromosomes such as those found in trisomy 21 (the cause of Down syndrome) or those correlated with some cancers. See CRISPR/Cas9 Deletes Whole Chromosome to learn more.
- For yet another twist on CRISPR power, check CRISPR-Based Gene ID in Fixed Cell Chromatin to read about a technique to identify and to locate specific DNA sequences in cells without disturbing chromatin structure.
Think of some examples of how CRISPR-based localization of a gene in intact chromatin might be useful.
As with any powerful technique (or powerful person for that matter!), behind the power as you might guess, lurks a dark side. The “dark side” of the CRISPR/Cas (and the related newer gene-editing technologies) was quickly recognized by its practitioners, most notably Jennifer Doudna herself. If for no other reason than its efficiency and simplicity, applications of CRISPR for precision gene editing has raised ethical issues. Clearly the potential exists for abuse or even for use with no beneficial purpose at all, and the availability of CRISPR kits only intensifies the concerns. We can be hopeful that, as in all discussions of biological ethics, scientists are very much engaged in the conversation. Listen to Doudna talk about CRISPR and its potential to do good in the world and its potential to be abused by the foolish and unscrupulous (Dr. Doudna Talks About CRISPR).
We will no doubt continue to edit genes with CRISPR-based technologies, But if you still have qualms, maybe RNA editing will be a useful alternative. Check out the link at Why Edit RNA? for an overview of the possibilities! As a final point, “mice and men” (and women and babies too) have antibodies to Cas9 proteins, suggesting prior exposure to microbial CRISPR/Cas9 antigens. This observation may limit clinical applications of the technology! See Uncertain Future of CRISPR/Cas9 Technology.
13.2.3 The Small RNAs: miRNA, siRNA and piRNA in Eukaryotes
Micro RNAs (miRNAs) and small interfering RNAs (siRNAs) are found in Caenorhabditis elegans, a small nematode (roundworm) that quickly became a model for studies of cell and molecular biology and development. You can see this roundworm in the fluorescence micrograph in Figure 13.5.

What are the attractions of C. elegans? Its ~21,700 genes are comparable to the ~25,000 genes in a human genome, it’s gene products produce an adult worm consisting of just 1,031 cells, these few cells are organized into all of the major organs found in higher organisms, and finally, it’s possible to trace the embryonic origins of each adult cell!
13.2.3.a Small Interfering RNA (siRNA)
siRNA was first found in plants and in C. elegans. But they are common in many higher organisms. siRNAs were so named because they interfere with the function of other RNAs foreign to the cell or organism. Their action was dubbed RNA interference (RNAi). For their discovery of siRNAs, A. Z. Fire and C. C. Mello shared the 2006 Nobel Prize in Physiology or Medicine. Figure 13.6 (below) illustrates the action of siRNA targeting foreign DNA.
When cells recognize foreign double stranded RNAs (e.g., some viral RNA genomes) as alien, a nuclease called dicer hydrolyzes them. The resulting short, double-stranded hydrolysis products (the siRNAs) combine with RNAi Induced Silencing Complex or RISC proteins. The antisense siRNA strand in the resulting siRNA-RISC complex then binds to complementary regions of foreign RNAs, targeting them for degradation. Cellular use of RISC to control gene expression in this way may have derived from the use of RISC proteins by miRNAs as part of a cellular defense mechanism, to be discussed next.
Custom-designed siRNAs have been used to disable the expression of specific genes to study their function in vivo and in vitro. Both siRNAs and miRNAs are being investigated as possible therapeutic tools to interfere with RNAs whose expression leads to cancer or other diseases.

234 siRNA Posttranscriptional Regulation
235 Did siRNA Co-opt RISC as a Strategy to Trash, Corrupt, or Wear Out RNA?
Check out the unexpected results of an experiment in which RNAi was used to block embryonic expression of the orthodenticle (odt) gene that is normally required for the growth of horns in a dung beetle. See the results at Genetically Modified Beetles Grow a Functional 3rd Eye. As expected, this knock-out mutation blocked horn growth. What was unexpected however was the development of an eye in the middle of the beetle’s head (the “third eye” in the micrograph). The third eye not only looks like an eye but also functions as one. This was demonstrated by preventing normal eye development in odt-knockout mutants. The third eye was responsive to light! Keep in mind that this was a beetle with a third eye, not Drosophila! Sometimes, especially with new technologies, we can learn as much by studying an unexpected critter as from a model research organism!
13.2.3.b Micro RNAs (miRNA)
miRNAs are widely distributed in eukaryotes, where they target unwanted endogenous cellular RNAs for degradation. After transcription, pre-miRNAs fold into hairpin loop structures that bind to protein complexes made up of DGCR8, RNAse III (among others), They are then processed into mature miRNAs. Like SiRNAs, mature miRNAs combine with RISC proteins. The RISC-protein/miRNA complex targets damaged or obsolete mRNAs. An estimated 250 miRNAs in humans may be sufficient to H-bond to diverse target RNAs; only targets with strong complementarity to a RISC-protein/miRNA complex will be degraded. Figure 13.7 shows the pathway from the transcription of a pre-miRNA, its processing, and ultimately to targeting unwanted mRNA for degradation.

236 miRNA Posttranscriptional Regulation
A recent study found that mutant embryonic stem cells (ESCs) unable to make the DGCR8 also fail to differentiate normal neurons, suggesting a role for miRNA in embryonic development. An experiment was designed to ask if the mutant DGCR8 protein disrupted development in some novel way, or just by inhibiting pre-mi-RNA processing. An miRNA look-alike (mi-R-302) was engineered that did not bind DGCR8 and thus, was not processed. When the mi-R-302 was introduced into the mutant ESCs, these cells went on to differentiate into neuronal cells, even though the look-alike molecule was not processed. confirming the involvement of miRNA in development.
miR-302 rescues mutant ESCs by bypassing the pre-miRNA processing step… Why then process miRNA in the first place!!?
13.2.3.c Piwi-Interacting RNAs
By some estimates, PIWI-Interacting RNA (piRNA) is the largest separate class of non-coding small RNA. It was first shown to be involved in destroying transposon transcripts to abort transposition. The basics of piRNA synthesis and action in D. melanogaster are illustrated in Figure 13.8 below.

The synthesis of piRNAs (follow the thick blue arrows) begins when the nascent primary piRNAs transcribed from a piRNA gene cluster associate with proteins. Those proteins remaining on the transcript after export to the cytoplasm are exchanged for a zucchini (Zuc) nuclease. Primary piRNA is then hydrolyzed to generate the mature piRNA. piRNA blocks transposition by two pathways (black arrows 1 and 2). In pathway 1, Zuc nuclease cleaves primary piRNA to make mature piRNA, which is picked up by the Piwi protein. The latter joins other proteins on the nascent transposon mRNA, activating Eggless (Egg), a histone methyltransferase. This enzyme catalyzes methylation of a histone to remodel chromatin to heterochromatin, thereby silencing further transposon mRNA transcription. In pathway 2, nascent transposon mRNA associates with proteins that target the mRNA for cytoplasmic destruction. In the cytoplasm, the transposon mRNA is captured by the Argonaute Aub (aubergine) protein by hybridization to complementary sequences in mature piRNA, a portion of which has already been transferred from Zuc to the AUB protein. The transposon mRNA is then digested, preventing actual transposition. Now, a remaining transposon mRNA fragment is transferred to Ago3 (Argonaute-3), where it can hybridize to complementary sequences in intact primary piRNA from Zuc and then digest it to mature piRNA. Aub and Ago-3 are part of a ping-pong cycle. The ping-pong cycle is an alternate source of piRNA for the Aub protein, while Zuc-mediated maturation of piRNA is the fork leading to the two pathways that block transposition. Like siRNAs and miRNAs, piRNAs also use a RISC pathway to degrade unwanted RNAs, notably in germline cells. Learn more about piRNA at PIWI-Interacting RNA and PIWI Proteins and PIWI-Interacting RNA.
13.2.4 Long Noncoding RNAs
Long noncoding RNAs (lncRNAs) are a yet another class of noncoding eukaryotic RNAs. The latter include transcripts of antisense, intronic, intergenic DNAs, as well as of pseudogenes and retroposons (pseudogenes are recognizable but mutant genes whose transcripts are nonfunctional; retroposons are one kind of transposon or mobile DNA element). While some lncRNAs might turn out to be incidental transcripts that the cell simply destroys, others have a role in regulating gene expression. A recently discovered lncRNA is XistAR, which—along with its gene product, Xist—is required to form Barr bodies. Recall that Barr bodies form in human females when one of the X chromosomes in somatic cells is inactivated. For a review of lncRNAs, see Lee, J. T. (2012. Epigenetic Regulation by Long Noncoding RNAs; Science 338, 1435-1439).
CRISPR editing of embryos might eventually be used to prevent the mutation forming the extra X chromosome of trisomy 21 (preventing Down syndrome). Explain how this could be done, in principle.
A recent article (at lncRNAs and smORFs). summarizes the discovery that some long noncoding RNAs contain short open reading frames (smORFs) that are actually translated into short peptides of at least thirty amino acids! Who knows? The human genome may indeed contain twenty-one thousand to twenty-five thousand (or more) protein-coding genes!
13.2.5 Circular RNAs (circRNA)
Though discovered more than twenty years ago, circular RNAs (circRNAs) are made in different eukaryotic cell types. Look at Circular RNAs-circRNA) to learn more about this peculiar result of alternative splicing. At first circRNAs were hard to isolate. When they were isolated, they contained “scrambled” exonic sequences and were therefore thought to be nonfunctional errors of mRNA splicing. In fact, circRNAs are fairly stable. Their levels can rise and fall in patterns suggesting that they are functional molecules. Levels of one circRNA, called circRims1, rise specifically during neural development. In mice, other circRNAs accumulate during synapse formation, likely influencing how these neurons will ultimately develop and function.
Thus, circRNAs don’t appear to be “molecular mistakes.” In fact, errors occurring during their synthesis may be correlated with disease! Speculations about circRNA functions include roles in gene regulation, particularly regulation of the genes or mRNAs from which they themselves are derived.


