11.4: Breaking the Genetic Code
- Page ID
- 88964
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Marshall W. Nirenberg and J. Heinrich Matthaei decoded the first triplet. They fractionated E. coli as shown in Figure 11.4, and then identified which fractions were required for cell-free protein synthesis, i.e., in vitro translation.

Check out the original work in the classic paper by Nirenberg MW and Matthaei JH [(1961) The dependence of cell-free protein synthesis in E. coli upon naturally occurring or synthetic polyribo-nucleotides. Proc. Natl. Acad. Sci. USA 47:1588-1602]. Various combinations of the isolated cell fractions were added back together, along with amino acids (one of which was radioactive) and ATP (as an energy source). After the mixture underwent a short incubation, Nirenberg and Matthaei looked for the presence of high molecular weight radioactive proteins as evidence of in vitro translation.
They found that all four final fractions (1–4 in Figure 11.4) must be added together to make radioactive proteins in the test tube. One of the essential cell fractions consisted of RNA that had been gently extracted from ribosomes (fraction 2 in the illustration). Reasoning that this RNA might be mRNA, they substituted a synthetic poly(U) preparation for this fraction in their cell-free protein-synthesizing mix, expecting poly(U) to encode a simple repeating amino acid. Nirenberg and Matthaei set up twenty reaction tubes, with a different amino acid in each… and made only polyphenylalanine! (Figure 11.5).
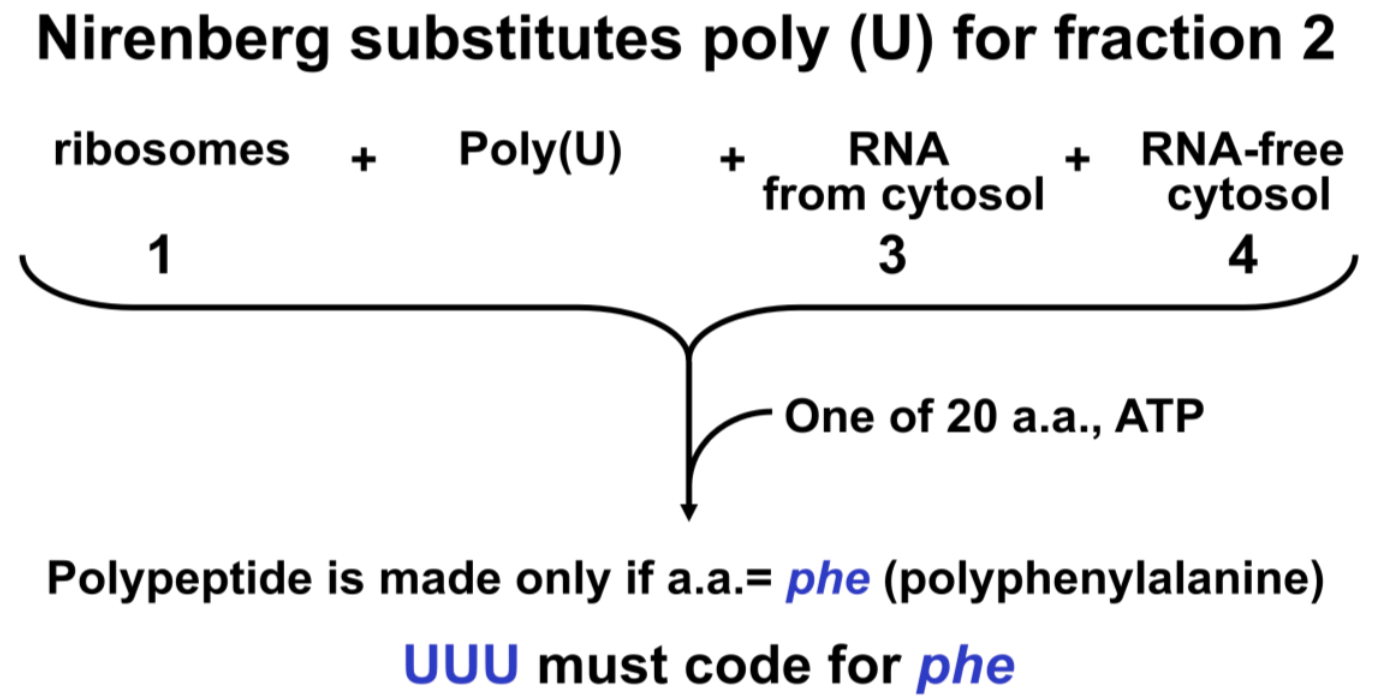
So the triplet codon UUU means phenylalanine. When other polynucleotides were synthesized by H. G. Khorana, poly(A) and poly(C) were shown in quick succession to make polylysine and polyproline using this experimental protocol. Thus, AAA and CCC must encode lysine and proline respectively. With much ingenuity and despite a bit more difficulty, additional codons were deciphered using poly di- and tri-nucleotides in cell-free systems.
203 Deciphering the First Codon
Nirenberg and Khorana shared the 1968 Nobel Prize in Physiology or Medicine for with R. W. Holley their contributions to our understanding of protein synthesis (see Holley’s contribution below).
The process of deciphering the rest of the genetic code relied on Crick’s realization that, chemically, amino acids have no attraction for either DNA or RNA (or triplets thereof). Instead, he predicted the existence of an adaptor molecule that would contain nucleic acid and amino acid information on the same molecule. Today we recognize this molecule as tRNA, the genetic decoding device.
Nirenberg and Philip Leder designed the experiment that pretty much broke the rest of the genetic code. They added individual amino acids to separate test tubes containing tRNAs, in effect causing the synthesis of specific aminoacyl-tRNAs. They then mixed their amino acid– bound tRNAs with isolated ribosomes and synthetic triplet codons. They had previously shown that synthetic three-nucleotide fragments would bind to ribosomes, so they hypothesized that triplet-bound ribosomes would in turn, bind appropriate amino acid–bound tRNAs. Figure 11.6 summarizes their experiment.

In this experiment, various combinations of tRNA, ribosomes, and aminoacyl-tRNAs were placed over a filter. Nirenberg and Leder knew that aminoacyl tRNAs alone passed through the filter and that ribosomes did not. They predicted that triplets would associate with the ribosomes, that this complex would bind the tRNA with the amino acid encoded by the bound triplet, and that the filters would retain this three-part complex, allowing identification of the amino acid retained on the filter. They would then be able to match each amino acid to a triplet codon(s). Ultimately, sixty-one of the codons were matched to specific amino acids: Since 61 codons specify 20 amino acids, the genetic code was degenerate!
204 Deciphering All 64 Triplet Codons
After the code was largely deciphered, Robert Holley sequenced and characterized a yeast tRNA—which is what earned him his share of the Nobel Prize (above). This first successful sequencing of a nucleic acid was possible because the RNA was short and contained several modified bases that facilitated the sequencing chemistry. Holley found the amino acid alanine at one end of his tRNA, and he found one of the anticodons for an alanine codon roughly in the middle of the tRNA sequence. From regions of internal sequence complementarity, Holley predicted that this (and other) tRNAs would fold and assume a stemloop (or cloverleaf) structure with a central anticodon loop. Figure 11.7 shows the stem-loop structure of a phenylalanine tRNA and a subsequent computer-generated the molecular structure with a now-familiar “L”-shape). Note the amino acid attachment site at the 3’ end at the top of the molecule and the anticodon loop at the bottom.

205-2 tRNA Structure and Base Modifications
After a brief overview of translation, we’ll break this process down into its three steps and see how aminoacyl-tRNAs function in the initiation and elongation steps of translation, and we’ll look at the special role of an initiator tRNA.


