9.4: The Process of Replication
- Page ID
- 88950
As noted, DNA replication is a sequence of repeated condensation (dehydration synthesis) reactions linking nucleotide monomers into a DNA polymer. Replication, like all biological polymerizations, proceeds in three enzymatically catalyzed and coordinated steps: initiation, elongation, and termination.
9.4.1. Initiation
As we have seen, DNA synthesis starts at one or more origins of replication. These are DNA sequences targeted by initiation proteins (Figure 9.7).
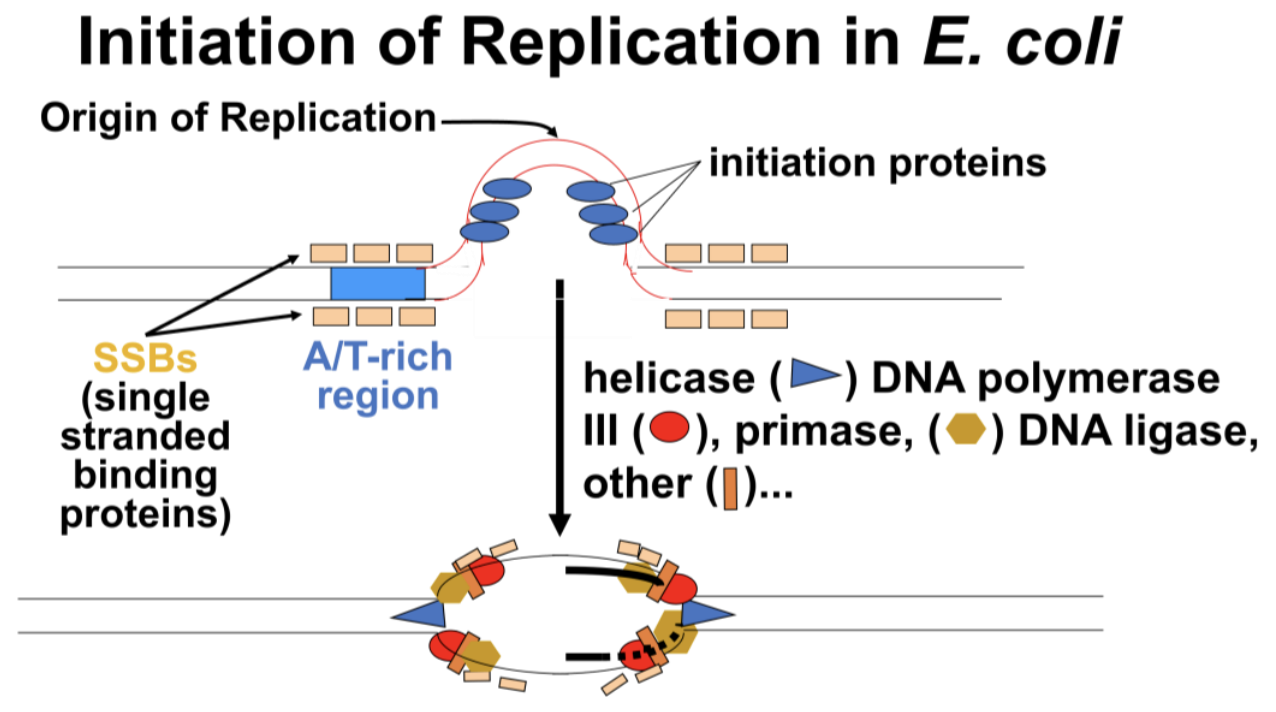
After these proteins break the hydrogen (H-) bonds at the origin of replication, the DNA double helix is progressively unzipped in both directions (i.e., by bidirectional replication). The separated DNA strands serve as templates for new DNA synthesis. Sequences at replication origins that bind to initiation proteins tend to be rich in adenine and thymine bases. This is because A-T base pairs have two H-bonds, which require less energy to break than the three H-bonds that hold G-C pairs together. Once initiation proteins loosen H-bonds at a replication origin, DNA helicase uses the energy of ATP hydrolysis to further unwind the double helix. DNA polymerase III is the main enzyme that then elongates new DNA. Once initiated, a replication bubble (replicon) forms as repeated cycles of elongation proceed at opposite replication forks.
179 Replication Initiation in E. Coli
Recall that new nucleotides are only added to the free 3’ hydroxyl end of a preexisting nucleic acid strand. Since no known DNA polymerase can start synthesizing new DNA strands from scratch, this is a problem!
of Why might the evolution of replication have led to strand-elongation that adds only to the 3′ end of a growing strand?
DNA polymerases require a primer, a nucleic acid strand onto which they can add nucleotides. So what is that primer, and where does it come from? RNA polymerases catalyze RNA synthesis from in a 5’-to-3’ direction and are the only polymerases that grow a new nucleic acid strand from the first base). So it was suggested that primers for replication might actually be RNA. Discovery of short stretches of RNA at the 5′ end of Okazaki fragments confirmed the notion of RNA primers. We now know that cells use a special RNA polymerase called primase, to make RNA primers against DNA templates. Replication from the 3’ end of a primer implies that DNA polymerases are able to add deoxynucleotides to the 3’ end of the RNA. We’ll see in the next section that the requirement for RNA primers is nowhere more evident than in the events at a replication fork.
9.4.2. Elongation
Looking at elongation at one replication fork, we see another problem: one of the two new DNA strands will grow continuously toward the replication fork as the double helix unwinds. But what about the other strand? Either this other strand must grow in pieces in the opposite direction, or it must wait to begin synthesis until the double helix is fully unwound. The problem is illustrated in Figure 9.8.

If one strand of DNA must be replicated in fragments, then those fragments would have to be stitched (i.e., ligated) together, as hypothesized in Figure 9.9.

According to this hypothesis, a new leading strand of DNA grows (is lengthened) continuously by sequential addition of nucleotides to its 3′ end, against its leading-strand template. The other strand, however, would be made in pieces that would be joined in phosphodiester linkages in a subsequent reaction (discontinuous replication). Because joining these new DNA fragments should take extra time, this new DNA is called the lagging strand, making its template the lagging-strand template.
Reiji Okazaki and his colleagues were studying infections of slow-growing mutants of T4 phage in E. coli host cells. A DNA ligase enzyme was already known to catalyze the circularization of the linear phage DNA molecules that were being replicated in infected host cells. Okazaki’s hypothesis was that a deficient DNA ligase in the mutant phage not only slowed down the circularization of the replicating T4 phage DNA but would also slow the joining phage DNA fragments replicated against at least one of the two template DNA strands.
The investigators compared the growth rates of wild-type and mutant T4 phage and demonstrated that slow growth of the mutant phage was due to a deficient DNA ligase enzyme. (Figure 9.10).

When they tested the hypothesis, they found that short DNA fragments did indeed accumulate in E. coli cells infected with ligase-deficient mutants but not in cells infected with wild type phage. The lagging strand fragments are now called Okazaki fragments. You can check out Reiji Okazaki’s original research at 1968 Okazaki article.
180-2 Okazaki Experiments: Solving a Problem at an RF
181 Okazaki Fragments Are Made Beginning with RNA Primers
Each Okazaki fragment would have to begin with a 5’ RNA primer, creating yet another dilemma! The RNA primer must be replaced with deoxynucleotides before the fragments could be stitched together—a process that indeed happens (Figure 9.11, below). Removal of RNA primer nucleotides from Okazaki fragments requires the action of DNA polymerase I.
This is the slow-acting DNA polymerase first characterized by Arthur Kornberg. DNA polymerase I has the unique ability to catalyze hydrolysis of the phosphodiester linkages between the RNA (or DNA) nucleotides and the 5’ end of a nucleic acid strand.

Another enzyme, flap endonuclease 1 (FEN1) plays a role in removing “flaps” of nucleic acid from the 5’ ends of the fragments. These flaps are often displaced by polymerase as the enzyme replaces the replication primer. At the same time as the RNA nucleotides are removed, DNA polymerase I catalyzes their replacement by appropriate deoxynucleotides
Finally, when a fragment is composed entirely of DNA, DNA ligase links it to the rest of the already-assembled lagging-strand DNA. Because of its 5’ exonuclease activity (not found in other DNA polymerases), DNA polymerase I also plays unique roles in DNA repair (discussed further in section 9.5).
As Cairns suggested and as others had demonstrated, replication proceeds in two directions from the origin and forms a replicon with two replication forks (RFs). Each RF has a primase associated with replicating Okazaki fragments along lagging strand templates. Figure 9.12 below illustrates the requirement for primases at replication forks.

Now we can ask what happens when replicons reach the ends of linear chromosomes in eukaryotes.
9.4.3. Termination
In prokaryotes, replication is complete when two replication forks meet after replicating their portion of the circular DNA molecule. In eukaryotes, many replicons fuse to become larger replicons, eventually reaching the ends of the chromosomes. And now there is still another problem, illustrated in Figure 9.13!

When a replicon nears the end of a double-stranded DNA molecule (i.e., the end of a chromosome), the new continuously synthesized strand stops when it reaches the 5’ end of its template DNA, and the primer is removed, having completed its replication. But what about lagging-strand replication? The illustration shows primer removal from an Okazaki fragment primed near the end of the chromosome, and replacement with DNA nucleotides catalyzed by DNA polymerase I. The question marks above the DNA point to a dilemma: if a final Okazaki fragment is primed and synthesized, DNA polymerase I has no free 3’ end to begin RNA nucleotide replacement with DNA nucleotides. The problem then would be that every time a cell replicates, at least one strand of new DNA would get shorter. Of course, this would not do—and doesn’t happen! Eukaryotic replication undergoes a termination process that extends the length of one of the two strands using the enzyme telomerase, as illustrated in Figure 9.14.

Telomerase consists of several proteins and an RNA molecule. From the drawing, the RNA component serves as a template for 5′→ 3’ extension of the problematic DNA strand. The protein with the requisite reverse transcriptase activity is called Telomerase Reverse Transcriptase, or TERT. The Telomerase RNA Component is called TERC. Carol Greider, Jack Szostak, and Elizabeth Blackburn shared the 2009 Nobel Prize in Physiology or Medicine for discovering telomerase.
183 Telomere Replication Prevents Chromosome Shortening
We know now that differentiated, non-dividing cells no longer produce the telomerase enzyme but that telomerase genes are still active in dividing cells (e.g., stem cells and cancer cells), which contain abundant telomerase.
Why the difference in telomerase activities between the two cell types here!? What is the connection between telomerase action and the Hayflick Limit? And… what is the Hayflick Limit
9.4.4 Is Replication Processive?
Drawings of replicons and replication forks suggest separate events on each DNA strand. Yet events at replication forks seem to be coordinated. Thus, replication may be processive, meaning both new DNA strands are replicated in the same direction at the same time, smoothing out the process. How might this be possible? The drawing in Figure 9.15 shows how lagging-strand template DNA bends so that it points in the same direction as the leading strand at the replication fork.

The replisome structure cartooned at the replication fork consists of clamp proteins, primase, helicase, DNA polymerase, and single-stranded binding proteins, among others. Now, newer techniques of visualizing replication by real-time fluorescence videography have called the processive model into question, suggesting that the replication process is anything but smooth! Is the observed jerky movement of DNA elongation in the video an artifact? Or is the model of smooth, coordinated replication integrated at a replisome no longer valid? And if not, must coordination of replication be defined and achieved in some other way? Or, finally, are lagging-strand and leading-strand replication simply just not coordinated? Check out the video yourself at Real-Time Fluorescent Replication Video.
9.4.5. One More Problem with Replication
Cairns recorded many images of E. coli of the sort shown in Figure 9.16.

The coiled, twisted appearance of the replicating circles was interpreted to be a natural consequence of the helically intertwined strands of DNA pulling apart (as is the case with intertwined strands of any material). As the strands continued to unwind, the DNA would twist into a supercoil of DNA. Too much unwinding would cause the phosphodiester linkages in the DNA to rupture, fragmenting the DNA. Obviously, this does not happen.
Experiments were devised to demonstrate supercoiling and to test hypotheses explaining how cells would relax the supercoils during replication. Testing these hypotheses revealed the topoisomerase enzymes. These enzymes bind and hold on to DNA, catalyze the hydrolysis of phosphodiester linkages, control the unwinding of the double helix, and finally catalyze the reformation of the phosphodiester linkages. It is important to note that the topoisomerases are not part of a replisome and can act far from a replication fork, probably responding to tensions anywhere in the supercoiled DNA. Recall that topoisomerases comprise much of the protein lying along eukaryotic chromatin.
185-2 Topoisomerase Relieve Supercoiling During Replication
We have considered most of the molecular players in replication. Key replication proteins and their functions are listed in Table 9.2 below, taken from DNA Replication.
Table 9.2
| ENZYME | FUNCTION IN DNA REPLICATION |
|---|---|
| DNA helicase | A helix destabilizing protein that unwinds a double helix at replication forks |
| DNA polyerase | Builds new double-stranded DNA, adding deoxy-nucleotides 5’-3’; some can proofread and correct errors |
| DNA clamp protein | Prevents DNA pol III from separating from the parent template strand. |
| Single-strand binding proteins (SSBs) | Keep unwound DNA strands at replication forks from reannealing during replication |
| topoisomerases | Relax supercoiled DNA caused by DNA unwinding during replication |
| DNA gyrases | A specific kind of topoisomerase |
| DNA ligase | Joins Okazaki Fragments to growing DNA strands during replication |
| primase | Initiates replication using nucleotides to synthesize an RNA primer required for DNA polymerases to then add deoxynucleotides |
| telomerase | Enzyme that adds repetitive DNA sequences to telomeric DNA to maintain the length of eukaryotic chromosomal DNA |


