6.5: Gluconeoqenesis
- Page ID
- 88928
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)In well-fed animals, most cells can store some of glucose as glycogen, which they break down as needed to retrieve nutrient energy as G-6-P. Glycogen hydrolysis (glycogenolysis) produces G-1-P, which is converted to G-6-P as we saw at the beginning of Stage 1 of glycolysis. But glycogen in most cells is quickly used up between meals. Therefore, most cells depend on a different, external source of carbohydrates (i.e., glucose) other than diet. Those sources are liver (and to a lesser extent kidney) that can store large amounts of glycogen after meals. In continual feeders (cows and other ruminants), glycogenolysis is ongoing.
Glycogenolysis by liver cells supplies glucose to the blood for up to six to eight hours between meals in intermittent feeders (like us), to be distributed as needed to all cells of the body. Thus, you can expect to use up liver and kidney glycogen reserves after a good night’s sleep, a period of intense exercise, or any prolonged period of low carbohydrate intake (fasting or starvation). Under these circumstances, animals use gluconeogenesis (literally, new glucose synthesis) in liver and kidney cells to provide systemic glucose to nourish other cells.
In healthy individuals, the hormones glucagon and insulin regulate blood glucose homeostasis, depending on cellular glucose (energy) needs. These hormones raise or lower blood glucose levels to protect the organism from hypoglycemia (low blood sugar) and hyperglycemia (high blood sugar), respectively. The gluconeogenic pathway produces glucose from carbohydrate and non-carbohydrate precursors that include pyruvate, lactate, glycerol, and gluconeogenic amino acids. The latter are amino acids that can be converted to alanine. The reactions of glycolysis and gluconeogenesis are shown side-by-side in Figure 6.18.

In Figure 6.18, look for bypass reactions that are catalyzed by carboxylases and phosphatases and the glycolytic reactions that function in reverse during gluconeogenesis. If glycolysis is an exergonic pathway, then gluconeogenesis must be an endergonic one. In fact, while glycolysis generates a net of two ATPs from two pyruvates, making glucose from two pyruvates during gluconeogenesis in contrast, costs four ATPs and two GTPs!
Likewise, gluconeogenesis is only possible if the bypass enzymes are present. These are necessary to get around the three biologically irreversible reactions of glycolysis. Except for the bypass reactions, gluconeogenesis is essentially a reversal of glycolysis.
Where do the nucleotide triphosphates (ATP and GTP) necessary to fuel gluconeogenesis come from?
As drawn in the pathways in Figure 6.18, glycolysis and gluconeogenesis would seem to be cyclic. In fact, this apparent cycle was recognized by Carl and Gerty Cori, who shared the 1947 Nobel Prize for Medicine or Physiology with Bernardo Houssay for discovering how glycogen is broken down in muscle cells (and in fact in most cells) to pyruvate to be reconverted to glucose in liver cells. Named after the Coris, The Cori cycle (Figure 6.19) recognizes the interdependence of liver and muscle in glucose breakdown and re-synthesis.

Despite the free energy requirement of gluconeogenic reactions under standard conditions (in a closed system), active gluconeogenesis in cells liver and kidney cells is energetically favorable! This is because the cells are open systems. The accumulation of pyruvate in liver cells and a rapid release of new glucose into the blood drive the now energetically favorable reactions of gluconeogenesis forward. Therefore, glucose synthesis occurs under gluconeogenic conditions with a negative \(\Delta G′\), a decline in actual free energy. Of course, glycolysis and gluconeogenesis are not simultaneous. Which pathways operate in which cells is tightly controlled. Glycolysis is the norm in all cell types, even liver and kidney. However, the cessation of glycolysis in favor of gluconeogenesis in the latter cells is under hormonal control (Figure 6.20).
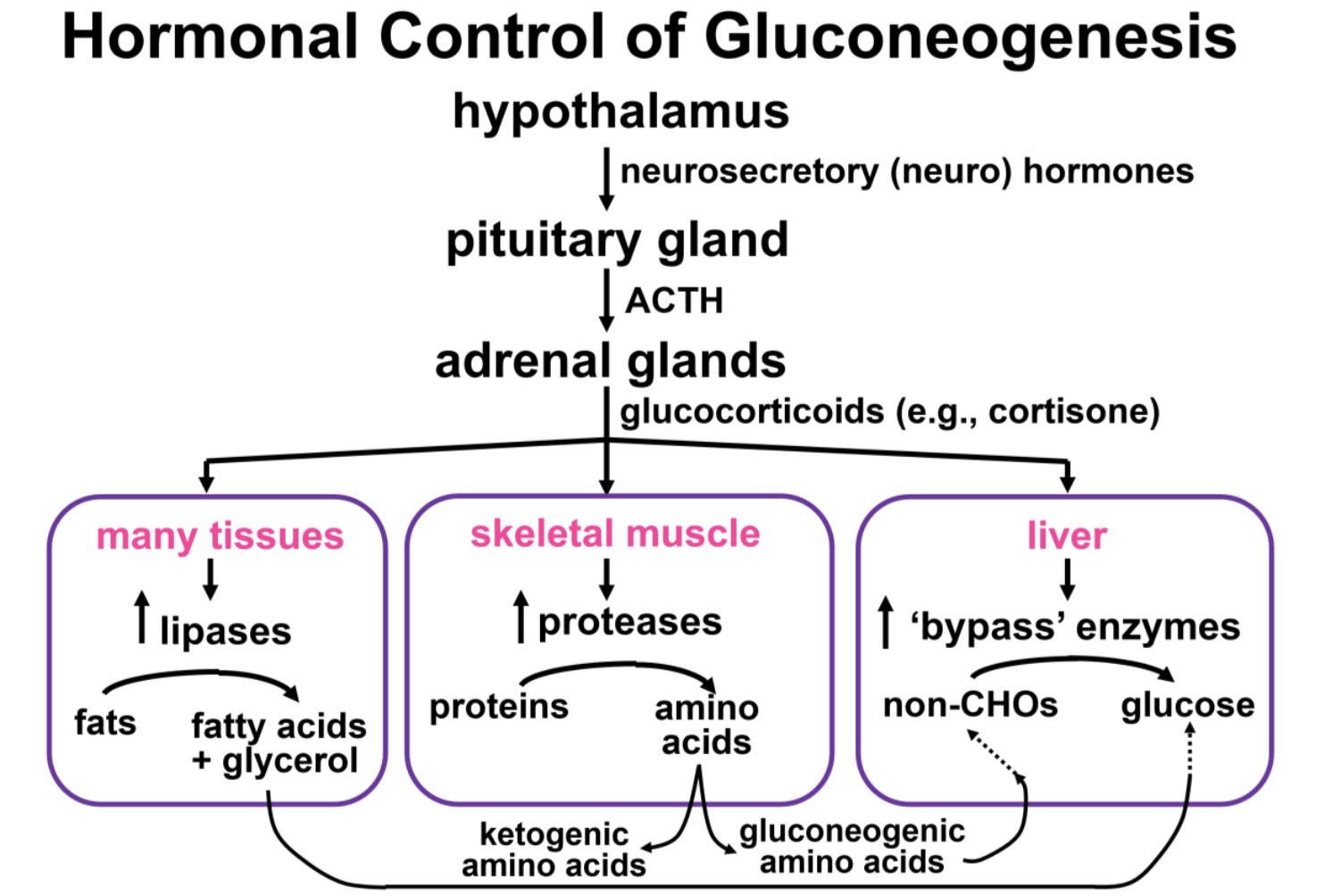
Key in turning on liver gluconeogenesis is the role of glucocorticoid hormones. What causes the secretion of glucocorticoids? A long night’s sleep, fasting and in the extreme, and starvation are forms of stress. Stress responses start in the hypothalamic-pituitary axis. Different stressors cause the hypothalamus to secrete a neurohormone which stimulates the release of ACTH (adrenocorticotropic hormone) from the pituitary gland.
ACTH then stimulates the release of cortisone and other glucocorticoids from the cortex (outer layer) of the adrenal glands. As the name glucocorticoid suggests, these hormones participate in the regulation of glucose metabolism.
Here is what happens at times of low blood sugar (e.g., when carbohydrate intake is low):
- Glucocorticoids stimulate the synthesis of gluconeogenic bypass enzymes in liver cells.
- Glucocorticoids stimulate protease synthesis in skeletal muscle, causing hydrolysis of the peptide bonds between amino acids. Gluconeogenic amino acids circulate to the liver where they are converted to pyruvate, a major precursor of gluconeogenesis. Some amino acids are ketogenic and are converted to Acetyl-S-CoA, a precursor to ketone bodies.
- Glucocorticoids stimulate increased levels of enzymes including lipases which catalyze hydrolysis of the ester linkages in triglycerides (fat) in adipose and other cells. This generates fatty acids and glycerol.
- Glycerol circulates to liver cells which take it up and convert it to G-3-P, augmenting gluconeogenesis. Fatty acids circulate to liver cells where they are oxidized to Acetyl-SCoA that is then converted to ketone bodies. and released to the circulation.
- Most cells switch from glycolysis to fatty acid oxidation an alternate energy source when glucose is limiting. Heart and brain cells depend on glucose for energy, but under extreme conditions (prolonged fasting, starvation), brain cells can use ketone bodies as an energy source of last resort.
Thus, the essential roles of glucocorticoids include the following:
- Enabling most cells to oxidize fats (fatty acids) for energy
- Allowing brain cells to use gluconeogenic glucose for energy, and in the extreme, ketone bodies as an alternate energy source
- Allowing cardiac muscle to use gluconeogenic glucose as its energy source
It’s a pity that we humans can’t use fatty acids as gluconeogenic substrates! Plants and some lower animals have a glyoxylate cycle pathway that can convert fatty-acid oxidation products directly into gluconeogenic carbohydrate substrates. Lacking this pathway, we (and higher animals in general) cannot convert fats to carbohydrates, in spite of the fact that we can all too easily convert the latter to the former! For us, when the gluconeogenic response is inadequate to the task, the body can resort to ketogenic fat metabolism. Think of this as a last resort, leading to the production of ketone bodies and the “acetone breath” in long-term fasting or people with severe eating disorders (e.g., anorexia nervosa).
The dark side of the limits of gluconeogenic metabolism is prolonged starvation, which will eventually overwhelm the gluconeogenic response. You see this in reports from third world regions where many people may suffer starvation due to drought, other natural disasters, or war. The spindly arms and legs of starving children result from muscle wasting as the body tries to provide the glucose necessary for survival.


