The Metric System and Measurement
- Page ID
- 2845
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Introduction
The metric system is the world standard for measurement. Not only is it used by scientists throughout the world, but most nations have adopted it as their standard of measurement. All of the measurements done in this course will use the metric system.
The table below shows the standard unit of length, mass, volume, and temperature in the metric system. It also shows the English equivalent.
| Metric | English | |
|---|---|---|
| Length | meter | 39.37 inches |
| Mass | gram | 0.03527 ounces |
| Volume | liter | 1.0567 quarts |
| Temperature | degree (Celsius) | 1.8 degrees Fahrenheit |
Meters, grams, and liters (see the table above) form the basis for larger or smaller units. The units are named using these prefixes:
Tera = 1,000,000,000,000
Giga = 1,000,000,000
Mega = 1,000,000
Kilo = 1,000
Hecto = 100
Deca = 10
Deci = 1/10
Centi = 1/100
Milli = 1/1,000
Micro = 1/1,000,000
Nano = 1/1,000,000,000
Pico = 1/1,000,000,000,000
The table below shows how meters are related to five other measures of length.
| Unit | Length |
| kilometer (km) | 1,000 m (1 × 103 m) |
| meter (m) | 1 m |
| centimeter (cm) | 0.01 m (1 × 10-2 m) |
| millimeter (mm) | 0.001 m (1 × 10-3 m) |
| micrometer (um) | 0.000001 m (1 × 10-6 m) |
| nanometer (nm) | 0.000000001 m (1 × 10-9 m) |
Notice that each of the units in the table above are related to meters by a multiple of 10.
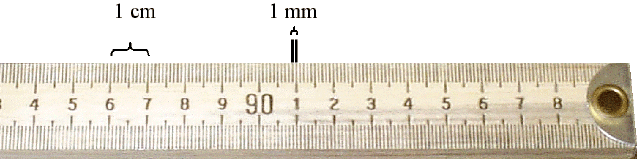
The photograph below shows the end of a meter stick. The 90 cm mark can be seen in the center of the photograph. One meter = 100 cm. Notice that each centimeter is divided into 10 mm.
The tables below show similar units based on grams (mass) and liters (volume).
| Unit | Mass |
| metric ton (t) | 1,000 kg or 1,000,000 g (1 × 106 g) |
| Kilogram (kg) | 1,000 g (1 × 103 g) |
| gram (g) | 1 gram |
| milligram (mg) | 0.001 g (1 × 10-3 g) |
| microgram (ug) | 0.000001 g (1 × 10-6 g) |
| nanogram (ng) | 0.000000001 g (1 × 10-9 g) |
| Unit | Volume |
| kiloliter (kl) | 1,000 liters (1 × 103 l) |
| liter (l) | 1 liter |
| milliliter (ml) | 0.001 liter (1 × 10-3 l), 1cm3 |
| microliter (ul) | 0.000001 liter (1 × 10-6 l) |
Notice in the table above that one milliliter (ml) equals one cubic centimeter (1 ml = 1 cc or cm3).
Metric Conversions
Exponents
The table below shows how numbers can be written using exponents. For example, a second way to write the number 1,000 is 1 × 103.
100 = 1
100 = 1 × 102
1000 = 1 × 103
10,000 = 1 × 104
0.01 = 1 × 10-2
0.001 = 1 × 10-3
Examples
256 = 2.56 × 102
3287 = 3.287 × 103
0.055 = 5.5 × 10-2
Exponents are useful when writing numbers that are very large or very small. For example the number 1,930,000,000,000,000,000 is easier to write as 1.93 × 1018.
Decimal Point
Metric conversions are done by moving the decimal point. When converting a large unit such as meters to a smaller unit such as millimeters, the decimal point is moved to the right. When converting smaller units to larger units, the decimal point is moved to the left. You must subtract the exponents in order to determine how many places to move the decimal point.
Larger unit - move decimal point to the left to make a smaller number
|
|
1012 |
tera (T) |
|
109 |
giga (G) |
|
|
106 |
mega (M) |
|
|
103 |
kilo (k) |
|
|
102 |
hecto (h) |
|
|
101 |
deca (da) |
|
|
100 |
||
|
10-1 |
deci |
|
|
10-2 |
centi (c) |
|
|
10-3 |
milli (m) |
|
|
10-6 |
micro (µ) |
|
|
10-9 |
nano (n) |
|
|
10-12 |
pico (p) |
Smaller unit - move decimal point to the right to make a larger number
Examples
Convert 2.6 cm to um.
This problem is solved by subtracting the exponents. The exponent for cm is -2; the exponent for um is -6. Subtract the two numbers: (-2 - (-6) = 4). Therefore, to convert 2.6 cm to um, you must move the decimal point 4 places to the right.
2.6 cm = 26000
Convert 57 um to cm.
The exponent for um is -6. The exponent for cm is -2. You must subtract these two number to determine how many places to move the decimal point. -6 - (-2) = -4. The negative sign indicates that you must move the decimal point 4 places to the left.
57 cm = 0.0057
Rounding
Several of the questions in this exercise ask you to round your answers. Rounding a number to the nearest 0.1 means that your answer should display one digit to the right of the decimal point. For example, the number 0.526 becomes 0.5. Similarly, rounding a number to the nearest 0.01 means that your answer should display two digits to the right of the decimal point. The number 0.526 rounded to the nearest 0.01 becomes 0.53. Notice that the 2 in 0.526 is rounded up to 3 (0.53) because the digit to the right of the 2 is 6. If the number to the right of the last digit being displayed is 5 or greater, the displayed number is increased by 1.
Examples
The number 0.4382251 rounded to the nearest 0.1 is 0.4.
The number 0.4382251 rounded to the nearest 0.01 is 0.44.
The number 0.4382251 rounded to the nearest 0.001 is 0.438.
The number 0.4382251 rounded to the nearest 0.0001 is 0.4382.
Laboratory Exercise
Record your answers to the questions below in your notebook. Do not use scientific notation (exponents) or fractions in your answers to the questions below. Write all of the zeros.
Length
Measurement of Length
Measure the width of a standard 8.5 × 11 inch page using a small plastic ruler or a meter stick. Record your measurement in 1) millimeters, 2) centimeters, and 3) meters. Record your answers on the answer sheet.
Use a meter stick to measure the width of the laboratory table as shown by the red line in the photograph below. Record your measurement in 4) millimeters, 5) centimeters, and 6) meters.
 |
Click the photograph to view an enlargement. |
7) Which unit of measurement (kilometer, meter, centimeter, millimeter, micrometer, or nanometer) would be most appropriate for measuring the width of this room?
Conversions of Length
Perform the following conversions.
8) 1 m = _____ cm.
9) 1 cm = _____ m.
10) 3.57 mm = _____ um.
11) 452 cm = _____ mm.
12) 0.04 um = _____ mm
13) 37.6 nm = _____ mm
14) 52 nm = _____ um
15) 0.05 um = _____ nm.
16) 4.3 m = _____ um
17) 4206 mm = _____ cm
18) 0.046 mm = _____ nm
19) 4.8 cm = _____ um
Use the following information to perform the calculations below.
Metric to English: 1 meter = 39.37008 inches = 3.28084 feet
English to Metric: 1 inch = 0.0254 meters; 1 foot = 0.3048 meters
20) 8.53 inches = _____ m Round your answer to the nearest 0.001 m.
21) 12 feet, 3 inches = _____ m Round your answer to the nearest 0.01 m. [Hint: First, convert 12 ft. 3 inches to feet. It is not 12.3 feet.]
Mass
Measurement of Mass
The laboratory scale shown below uses weight to determine mass. It has a sensitivity of 0.001 g. Due to its sensitivity, moving air will cause it to fluctuate. The glass chamber surrounding the weighing pan prevents air currents from interfering with the measurement.
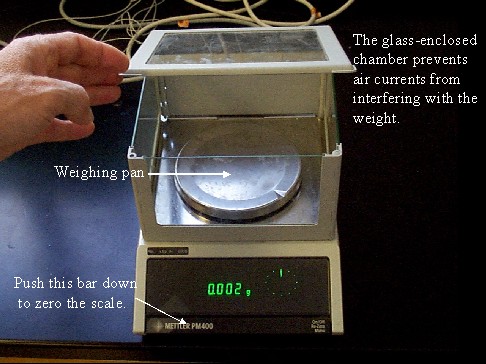
The scale in the photograph below has a sensitivity of 0.01 g. The scale can be set to zero by pressing the zero (tare) button on the lower left part of the scale.

Place a small beaker on the pan of the scale and zero it by pressing down on the zero (tare) button located on the front of the scale. Place a penny in the beaker to obtain its mass.
22) How much does the penny weigh in grams?
Remove the beaker from the scale and weigh the penny without using the beaker. You must first zero the scale before weighing the penny.
Conversions of Mass
Perform the following conversions.
23) 37 g = _____ mg
24) 0.047 mg = _____ g
25) 45.36 g = _____ kg
Use the following information to perform the calculations below.
Metric to English: 1 g = 0.0352739 ounces = 0.0022046 pounds
English to Metric: 1 ounce = 28.34951 grams; 1 pound = 453.5924 grams
26) 150 pounds = _____ kg Round your answer to the nearest 0.01 kg.
27) 3 oz = _____ g Round your answer to the nearest 0.01 g.
Volume
Measurement of Volume
28) Obtain a 10 ml graduated cylinder (shown below) and add some water to it. Hold the graduated cylinder in a vertical position at eye level and read the number of milliliters of water that are in the cylinder. Be sure to read the water at the bottom of the meniscus. The arrow points to the bottom of the meniscus in the photograph below. What is the volume of water in your cylinder?

29) Use a 50 or 100 ml graduated cylinder to determine the amount of liquid that a test tube can hold (it's volume).

How did you determine the volume of the test tube?
Conversions of Volume
30) 42 ml = _____ liters
31) 27 ul = _____ liters
32) 3.6 l = _____ ml
33) 1 ml = _____ ul
Sometimes volume is measured using cubic centimeters (abbreviated cc or cm3). One cubic centimeter equals one milliliter (1cc = 1ml).
34) 27 liters = _____ cc (or cm3)
Use the following information to perform the calculations below.
Metric to English: 1 liter = 1.056688 quarts = 0.2641721 gallons
English to Metric: 1 quart = 0.9463529 liters; 1 gallon = 3.785412 liters
35) 2.3 quarts = _____ liters Round your answer to the nearest 0.01 liter.
36) 0.5 gallons = _____ liters Round your answer to the nearest 0.01 liter.
Temperature
Measurement of Temperature
The following temperature measurements should be done in Celsius.
37) Measure and record the temperature of the air in the laboratory room.
38) Measure and record the temperature of ice water.
39) Measure and record the temperature of boiling water.
Conversions of Temperature
The temperature in Fahrenheit can be converted to Celsius using the formula:
\[\mathrm{°C = \dfrac{5}{9}(°F - 32)}\]
For example, to convert 60° F to ° C, subtract 32 (=28), multiply it by 5 (=140) and divide it by 9 (=15.56).
The steps listed above are performed in reverse order to convert Celsius to Fahrenheit. The equation is below:
\[\mathrm{°F = \left(\dfrac{9}{5}\: °C\right) + 32}\]
For example, 20° C is converted to ° F by multiplying it by 9 (= 180), dividing it by 5 (= 36), and adding 32 (=68).
40) 72° F = _____°C For this one, use the formula \(\mathrm{°C = \dfrac{5}{9}(°F - 32)}\). Round your answer to the nearest 0.1.
(Note- If you do not have a calculator, use the one on the computer. Click Start, Programs, Accessories, Calculator).
41) 37° C = _____°F For this one, use the formula \(\mathrm{°F = \left( \dfrac{9}{5}\: °C\right) + 32}\)