1.2: Osmosis Teacher's Preparation Notes
- Page ID
- 25183
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)This activity provides a sequence of learning activities designed to optimize student learning and understanding of osmosis by beginning with a student investigation of osmosis at the macroscopic level and then moving to analyzing osmosis at the molecular and cellular levels. In Part I, "What is happening to these eggs?" students observe and analyze the effects of osmosis on eggs. In Part II, "Osmosis – Effects on Animal and Plant Cells", analysis and discussion questions introduce students to a molecular and cellular understanding of osmosis and challenge students to apply their understanding of osmosis to several real-world phenomena.
Learning Goals
In accord with the Next Generation Science Standards:
- This activity helps students to prepare for the Performance Expectation:
- MS-LS1-2. "Develop and use a model to describe the function of a cell as a whole and ways parts of cells contribute to the function."
- Students learn one aspect of the Disciplinary Core Idea (LS1.A) "… the cell membrane forms the boundary that controls what enters and leaves the cell."
- Students engage in recommended Scientific Practices, including "… carrying out investigations", "interpreting data", and "constructing explanations and designing solutions".
- Discussion of this activity can incorporate two Crosscutting Concepts: "Cause and effect: Mechanism and explanation" and "Structure and function".
Specific Learning Goals include:
- A selectively permeable membrane allows some types of molecules or ions to pass through, but not others.
- In biological organisms, each cell is surrounded by a selectively permeable cell membrane which regulates what gets into and out of the cell. Water is a small molecule that readily crosses the selectively permeable cell membrane.
- Movement of water across a selectively permeable membrane is called osmosis; osmosis results in the net movement of water from a solution with a lower concentration of solutes to a solution with a higher concentration of solutes.
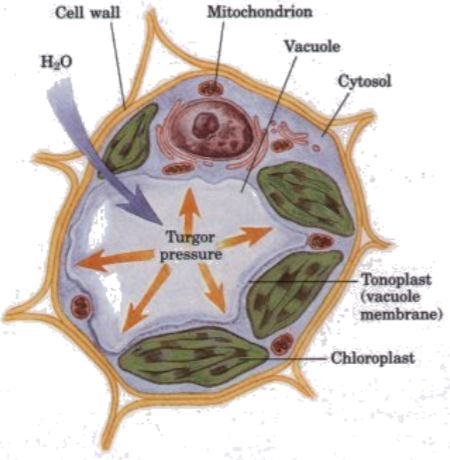
- If the solution surrounding a cell has a greater concentration of solutes than the cytosol inside the cell, there will be a net movement of water out of the cell. Conversely, if the solution surrounding a cell has a lower concentration of solutes than the cytosol inside the cell, there will be a net movement of water into the cell. This can cause animal cells to burst. However, in plant cells, the influx of water is limited by pressure from the surrounding cell wall.
Supplies
- For each student group (or just one set of supplies to prepare a demonstration):
- 2 eggs (To minimize the chance of salmonella infection you may want to use pasteurized eggs, in which case gloves would probably not be necessary.)
- 2 containers with covers (or plastic wrap)
- White vinegar (enough to cover each egg in its container)
- Corn syrup (enough to cover one egg in its container)
- Water (enough to cover one egg in its container, plus water to wash the corn syrup off of the egg on day 3)
- At least 3 gloves (An alternative that may be acceptable would be to have students wash their hands thoroughly each time after they have handled an egg.)
- Paper towels
- You will also need student access to a sink and either a scale with the container for weighing eggs and/or a measuring tape. Obviously, the activity will proceed more rapidly if you have more than one sink, scale and/or measuring tape.
Instructional Suggestions and Background Information
Part I will require approximately 15 minutes on three consecutive class days. Part II will probably require the rest of a 50-minute period on the third day. If you can't do Part I as a student investigation, you can present it as a demonstration, which will require less time.
A key is available upon request to Ingrid Waldron (iwaldron@sas.upenn.edu). The following paragraphs provide additional instructional suggestions and background information – some for inclusion in your class discussions and some to provide you with the relevant background that may be useful for your understanding and/or for responding to student questions.
I. What is Happening to These Eggs?
An unfertilized egg is a single cell, but a very atypical cell in size and makeup. Most of the egg yolk and white consist of storage molecules (mainly fat in the yolk and mainly protein in the white). In a fertilized, incubated egg these storage molecules are used during the development of the chick. The egg’s DNA is contained in a small spot on the surface of the yolk. The shell membranes that surround the egg cell membrane and lie just under the shell have protein fibers that give the shell membranes much greater strength than a cell membrane. The large size and strong shell membranes of a chicken egg make it useful for demonstrating osmosis, a process which also takes place in more typical cells, but is not as easy to observe.
Overall, a chicken egg is 74% water, which is similar to typical animal cells with ~70% water. White vinegar is about 95% water and 5% acetic acid. Corn syrup is about 20% water, and the rest is monosaccharides, disaccharides and oligosaccharides. A chicken egg has 88% water in the white and 48% water in the yolk. The higher concentration of water in the white is the major reason why, in an egg that has been in corn syrup, the white is much more reduced and the yolk becomes more prominent.
On Day 1, students can see bubbles forming as the acetic acid of the vinegar reacts with the calcium carbonate of the shell to produce CO2 bubbles:
2 CH3COOH + CaCO3 – > Ca(CH3COO)2 + H2CO3 – > Ca(CH3COO)2 + H2O + CO2
On Day 2, there will still be patches of shell on the surface of the eggs. It is possible to remove most of these patches of shell by washing while rubbing gently. However, this step is not necessary and there is a significant risk that students may break the shell membrane. The membranes surrounding the egg are relatively tough, but you do need to be gentle!
On Day 3, students will be able to see a dramatic difference in appearance between the enlarged egg that has been in the water and the shrunken, shriveled egg that has been in corn syrup. The water that diffuses out of the egg in corn syrup typically forms a layer of water on top of the corn syrup; this layer of water will be particularly obvious if you use dark corn syrup, but it is easier to see the shrunken egg if you use clear corn syrup.
You may want to relate the appearance of the egg in corn syrup to the use of salt to kill slugs by causing osmotic loss of water. It should be mentioned that salt in the soil is not good for plant health, so other approaches to getting rid of garden slugs are generally preferable. You may also want to mention the use of salt to preserve foods by making an environment that is too hypertonic for bacteria to survive.
If you modify this activity so your students are not making their own quantitative measurements, you may want to have the following sample data.
|
Day |
Egg 1 |
Egg 2 |
|||
|
Weight (grams) |
Circumference (cm) |
Weight (grams) |
Circumference |
||
|
1 |
56.3 (with shell) |
~14.0 |
52.6 (with shell) |
~13.9 cm |
|
|
Egg put into vinegar |
Egg put into vinegar |
||||
|
2 |
72.7 (without shell) |
~15.1 |
65.2 (without shell) |
~15.0 |
|
|
Egg put into water |
Egg put into corn syrup |
||||
|
3 |
83.5 |
~16.0 |
36.2 |
~9.9 |
|
II. Osmosis – Effects on Animal and Plant Cells
This section engages students in understanding the effects of osmosis on animal and plant cells. In addition, students use their understanding of osmosis to explain several real-world phenomena. You can use this section without the first section, but you would want to omit question 12 in the Student Handout since this question engages students in applying their understanding of osmosis to the results of the egg experiment.
The Student Handout does not introduce the terms hypertonic, hypotonic and isotonic (or hyperosmotic, hypo-osmotic and iso-osmotic), but you can add these terms if they are part of your learning goals for your students.
The molecular mechanism of osmosis is still controversial. The mechanism presented in most college biology textbooks is described below since it provides one way of understanding the phenomenon of osmosis. However, it should be noted that current evidence indicates that this is not the predominant mechanism responsible for osmosis.
Na+, Cl-, and the water molecules that are bound to these ions cannot cross the selectively permeable membrane. Only free water molecules (water molecules that are not bound to ions or other dissolved substances) can cross the selectively permeable membrane.

During osmosis, diffusion results in movement of free water molecules in both directions across the selectively permeable membrane, but more free water molecules move from the region of higher concentration of free water molecules to the region of lower concentration.
The concentration of free water molecules is lower in water with dissolved salt because some of the polar water molecules are attracted to the charged ions, so these water molecules are no longer free but are instead bound to water. Osmosis results in the net movement of water from the region of higher concentration of free water molecules (pure water) to the region of lower concentration of free water molecules (water with dissolved salt).

As mentioned above, current evidence indicates that this is not the most important molecular mechanism for osmosis.
For question 13, the contrast between animal and plant cells illustrates how adaptations are interrelated. Since animals cannot make their own food they generally need to move to get food, so rigid cell walls would be a disadvantage. Furthermore, for many animals, the function of the circulatory, respiratory and digestive systems depends on contractions of muscle cells, which is another reason why rigid cell walls would be a disadvantage.
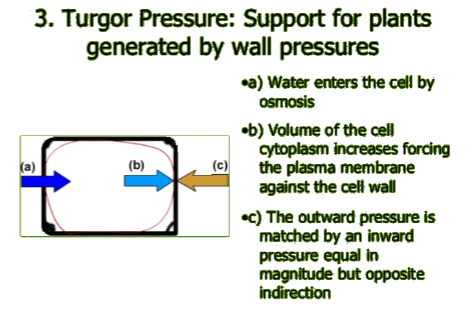
Sadie cells generally require isotonic surrounding extracellular fluid, whereas plant cells can flourish in hypotonic surrounding fluid. When there is little water in the soil, plant roots are unable to take up water, so the extracellular fluid becomes hypertonic and cells lose turgor pressure which is necessary to support the plant's structure. This is why a plant wilts if there is no rain or you forget to water the plant. In a hypotonic surrounding solution, the net flow of water into a plant cell will stop when enough water has entered the cell so the turgor pressure (b) and the countervailing cell wall pressure (c) match the solute potential (due to the greater solute concentration inside the cell). (For additional explanation, see http://media.collegeboard.com/digitalServices/pdf/ap/bio-manual/Bio_Lab4-DiffusionandOsmosis.pdf).
|
(http://www.bioinfo.org.cn/book/biochemistry/chapt02/35-1.jpg ) |
|
Challenge Questions
When a person rapidly consumes large quantities of water, this can result in hypotonic extracellular fluid, because the influx of water is too rapid to allow the normal osmotic regulation by the kidneys. The consequent osmotic effects can result in swollen cells, with the most harmful effects on the brain cells, which are especially prone to malfunction due to increased mechanical pressure as the swollen brain cells press against the skull. Hyponatremia (low concentration of sodium) and water intoxication can be fatal. This has been observed in some marathon runners who have consumed excessive amounts of fluids; this can result from excessive consumption of either water or sports drinks, both of which are hypotonic relative to our bodies' extracellular fluids and sweat. This example illustrates that dose makes the poison; in other words, at high enough doses even a necessary and relatively innocuous molecule like water can become fatal. This example also illustrates that official advice (e.g. that athletes need to drink more fluids) can result in harmful outcomes if carried to extremes. The former official advice that athletes should drink "ahead of their thirst" is currently disputed as a result of recent evidence that optimum athletic performance and health is generally observed when athletes have free access to water and drink to thirst ("Dehydration and endurance performance in competitive athletes", Nutrition Reviews 70 (suppl. 2): S5132-6; http://serendip.brynmawr.edu/exchange/bioactivities/sportsdrinks).
Athletes who are very active in a hot environment can become dehydrated as a result of copious sweating, although this is less likely if they have the opportunity to drink when they are thirsty. Dehydration can result in a decreased volume of body fluids, including decreased blood volume. As a result, not enough blood returns to the heart and the heart can't pump enough blood to maintain adequate circulation, so blood pressure drops. This can result in decreased athletic performance and increased risk of heat cramps, heat exhaustion, and in extreme cases heat stroke. Thus, it is important to replace lost water and salt.
Someone who is stranded at sea without any water should not drink ocean water. Ocean water is saltier than the most concentrated urine that human kidneys can produce. Therefore, drinking ocean water will cause an increase in the salt concentration of the body's extracellular fluid, and the hypertonic extracellular fluid will result in dehydration of the body's cells.
The challenge question concerning archaea that are adapted to highly saline environments makes the point that different types of cells are adapted to different environments. This question challenges students to be creative in proposing how cells might adapt to highly saline environments. Many archaea that live in highly saline environments synthesize small organic molecules (e.g. sugars), and the high concentrations of these solutes inside the cell help to balance the high concentration of salt in the surrounding environment so the cell does not lose water by osmosis. This illustrates how osmosis depends on the total concentration of solutes, even when the specific solutes differ. Some halophilic archaea (e.g. Haloferax) have a different adaptation to highly saline environments; they pump potassium into the cell in order to maintain osmotic equilibrium; in these archaea, enzymes and structural cell components have special characteristics to allow them to function at high salt concentrations.
Students may propose other possible adaptations for living in extremely salty environments, and it will be enlightening to discuss their proposals. For example, students may propose having a cell membrane that is impermeable to water; this will provide the opportunity to discuss the problems that would result from having such an impermeable cell membrane. Students may also propose that archaea might pump water across the cell membrane into the cell to counteract the osmotic effects of the hypertonic surrounding solution; thus far, biologists have not found any biological molecule that can directly pump water; instead, water is moved across cell membranes by osmotic gradients which cells can set up by pumping ions.
Possible Follow-Up Activities
You may want to have your students carry out a follow-up experiment, using one of the following options:
- Analyze osmosis and movement of other molecules and ions across dialysis tubing; see "Diffusion across a Selectively Permeable Membrane" (available at http://serendip.brynmawr.edu/sci_edu/waldron/#diffusion).
- Analyze osmosis in Elodea or moss cells and across dialysis tubing; see "Diffusion and Osmosis" (available at http://media.collegeboard.com/digitalServices/pdf/ap/bio-manual/Bio_Lab4-DiffusionandOsmosis.pdf ).
- Analyze osmosis across dialysis tubing, in pieces of potato, and in onion skin; see "Osmosis and Diffusion" (available at http://www.biologyjunction.com/osmosis_lab_example_2.htm).
- Use microscopes, Elodea and various chemicals to study osmosis and rates of diffusion across the cell membrane of molecules of different size and hydrophobicity; see "Diffusion across Biological Membranes" (available at http://faculty.buffalostate.edu/wadswogj/courses/BIO211%20Page/lectures/lab%20pdf's/Diffusion%20lab%2006a.pdf ).
Another useful follow-up to this hands-on activity would be 8 in the computer simulation "Diffusion" (available at http://mw.concord.org/modeler/index.html). You will want to set the membrane to movable to get reasonable results.
Another possible follow-up activity would be Should you drink sports drinks? When? Why? (available at http://serendip.brynmawr.edu/exchange/bioactivities/sportsdrinks ). The questions in this activity help students to understand the effects of consuming sports drinks and when and how the consumption of sports drinks can be beneficial or harmful. This activity provides the opportunity to review some basic concepts related to osmosis, cellular respiration, mammalian temperature regulation, and how our different body systems cooperate to maintain homeostasis.