1.1: Osmosis Protocol
- Page ID
- 25181
I. What is Happening to These Eggs?
Each cell is surrounded by a cell membrane that regulates what gets into and out of the cell.
1. Why is it important for each cell to be surrounded by a cell membrane that can prevent large molecules from leaving the cell?
2. The cell membrane allows some small molecules like oxygen to cross. Why is it important for oxygen to be able to cross the cell membrane?
Most cells are tiny – much too small to see without the help of a microscope. In contrast, an unfertilized chicken egg is a giant cell. In this investigation, you will see that water can cross the cell membrane surrounding an egg. You will investigate which way water moves across the membrane, depending on the type of liquid surrounding the egg. When water moves across the cell membrane, the egg changes in size and appearance.
- Your group will be given two eggs. To begin, record the weight and/or circumference of these eggs in the day 1 row of the table. (Measure the circumference around the widest part, not lengthwise.)
Caution: Because these are raw eggs, they may carry salmonella, so you should use gloves when handling the eggs.
| Day | Egg 1 | Egg 2 | ||
| Weight (grams) | Circumference (cm) | Weight (grams) | Circumference (cm) | |
| 1 | (with shell) | (with shell) | ||
| Egg put into vinegar | Egg put into vinegar | |||
| 2 |
(most of shell removed) |
(most of shell removed) |
||
| Egg put into water | Egg put into water | |||
| 3 | ||||
- Put each egg in a container labeled Egg 1 or Egg 2 with enough vinegar to cover the egg. Cover the container. Do you see bubbles forming around the egg? These are bubbles of CO2 which result from the chemical reaction between the acetic acid in the vinegar and the calcium carbonate in the eggshell. This reaction will dissolve most of the eggshell by day 2.
Day 2
- Observe your eggs. Notice that most of the shell has been dissolved by the acetic acid in the vinegar. Although most of the shell is gone, each egg is still surrounded by a shell membrane outside the cell membrane. The shell membrane has protein fibers that give it much greater strength than the cell membrane. However, the egg without its shell is still fragile, so you will need to handle your eggs very gently and carefully!
- Dry each egg and measure the weight and/or circumference of each egg. Record your results for day 2 in the table on page 1.
3. Did the eggs became heavier/larger ___ or lighter/smaller ___?
What do you think happened to cause this change in weight/size?
- Empty the vinegar from the container for egg 1 and replace it with water to cover the egg.
- Empty the vinegar from the container for egg 2 and replace it with corn syrup to cover the egg. As you pour the corn syrup, notice that it is viscous (thick, sticky).
4. What do you think causes the corn syrup to be so viscous?
Day 3
5. Compare and contrast the appearance of the egg that has been in water vs. the egg that has been in corn syrup.
6. You may be able to see a layer of water on top of the corn syrup. Where do you think this water came from?
- Rinse the corn syrup off of egg 2. Dry each egg and measure and record the weight and/or circumference for day 3 in the table on page 1.
7. What do you think happened to cause the change in weight/size of the egg placed in corn syrup?
8. Why did the egg placed in the water get bigger and heavier? Where do you think the additional weight/volume came from?
II. Osmosis – Effects on Animal and Plant Cells
The cell membrane that surrounds each cell is a selectively permeable membrane. A selectively permeable membrane allows some types of molecules and ions to cross the membrane and prevents other types of molecules and ions from crossing the membrane.
Osmosis is the movement of water across a selectively permeable membrane. As a result of osmosis, there is a net movement of water from a solution with a lower concentration of solutes to a solution with a higher concentration of solutes.
Inside the cell membrane, each cell contains cytosol, a watery substance with a relatively high concentration of solutes (dissolved molecules and ions).
9. If a cell is placed in pure water, will there be: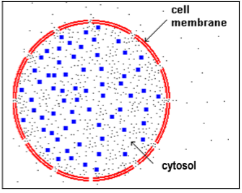
____ net movement of water into the cell?
____ net movement of water out of the cell?
____ no net movement of water into or out of the cell?
Explain your reasoning.
This figure shows the effects of osmosis on animal and plant cells put in three different types of the surrounding fluid.
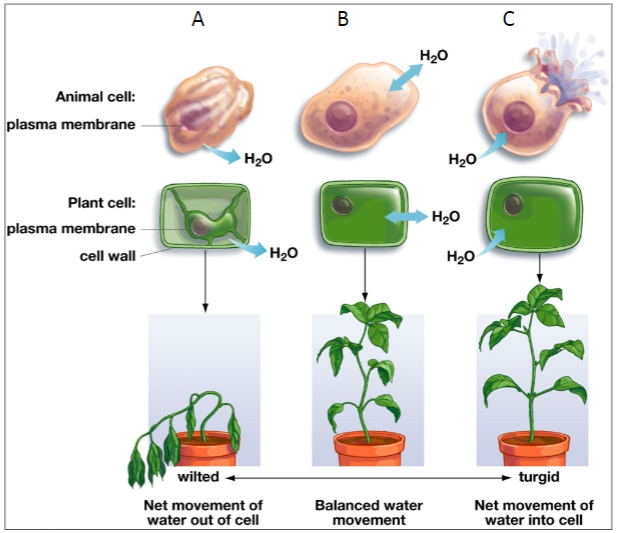
10a. Which of these diagrams shows the expected effect of putting a cell in pure water? ____
10b. Which of these diagrams shows the expected effect of putting a cell in very salty water which has a higher concentration of solutes than cytosol? ____
Explain your reasoning.
11. Most animal cells are surrounded by a layer of water with dissolved substances. For animal cells to function normally, there should be equal amounts of water diffusing into and out of the cell, as shown in figure B. Which type of surrounding fluid would result in equal amounts of water diffusing into and out of a cell?
____ water with a higher concentration of solutes than the cytosol
____ water with a lower concentration of solutes than the cytosol
____ water with the same concentration of solutes as the cytosol
12a. Which animal cell in the figure on the previous page looks like the egg in corn syrup? ____
12b. Which animal cell looks like what could have happened to the egg in water, if the egg didn't have a strong shell membrane around it? ____
12c. Based on the results of the egg experiment, which has a higher concentration of solutes – the contents of the egg ___ or corn syrup ___? How do you know?
12d. Based on the results of the egg experiment, which has a higher concentration of solutes – the contents of the egg ___ or vinegar ___? How do you know?
13a. Explain why the surrounding fluid has a different effect on animal cells vs. plant cells in figure C on the previous page.
13b. Suppose that an animal's cells had cell walls. What problems would this cause for the animal?
Challenge Questions
A. If a person drinks a very large amount of water in a short time without consuming any salt, this can result in confusion, seizures, coma, or even death, due to abnormal functioning of nerve cells in the brain. Explain how these problems could result from drinking too much water too rapidly.
B. What do you think is the reason that a person who is stranded at sea should not drink ocean water? How could drinking salty water harm a person's cells?
C. Some archaea (which are single-cell organisms) live in extremely salty water such as the Great Salt Lake or the Dead Sea. Most types of cells would shrivel and die in this very salty water. How do you think these archaea prevent water loss while living in very salty water?


