1.9: Evaluating the Ability of the ykkCD Toxin Sensor to Recognize the Antibiotic Tetracycline Using Fluorescent Quenching
- Page ID
- 18926
9.1 Learning Objective
The goal of the mini project was to identify elements in the toxin sensor that are essential to recognize the antibiotic tetracycline. You identified elements in the sensor that did not change throughout evolution (invariable blocks). You subjected them to the site-directed mutagenesis, modified the sequence of the toxin sensor DNA and made the mutated toxin sensor RNAs. During this lab you will evaluate how well this mutant sensor is able to recognize the antibiotic tetracycline. If the mutant sensor is still able to recognize tetracycline, it means that the mutated invariable block was not essential for sensory function. If however, the mutated sensor fails to recognize tetracycline, it means that the mutated invariable block was essential for sensory function. You will perform binding affinity assays using fluorescence quenching to evaluate the affinity of the mutant sensor toward tetracycline.
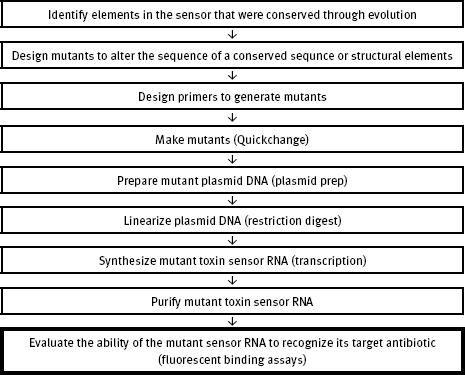
9.2 Mini Project Flowchart
The bolded block in the flowchart below highlights the role of the current experiment in the mini project.
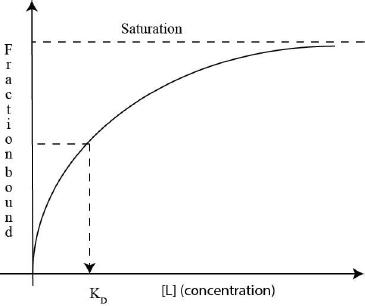
9.3 What is Binding Affinity (KD)?
To estimate how well the mutated sensor RNA recognizes the antibiotic tetracycline, you will measure the binding affinity (KD) of the tetracycline-sensor RNA complex. Binding affinity is the equilibrium dissociation constant. There is an inverse relationship between the strength of the interaction and the numeric value of the binding affinity: if the binding affinity is a small number, it means that addition of a small amount of RNA to tetracycline results in a high concentration of the sensor RNA-tetracycline complex. In other words, it only takes a small amount of tetracycline to form enough complex with the sensor RNA to trigger production of the efflux pump that in turn gets rid of tetracycline. Therefore, a small KD value reflects a strong interaction between the antibiotic tetracycline and the sensor RNA. Likewise, a large KD value reflects a week interaction between tetracycline and the sensor RNA. In other words if the KD value is large, it takes a large amount of tetracycline to form enough tetracycline-RNA complex to trigger bacterial defense that gets rid of tetracycline. To measure binding affinity, one of the two reactants (tetracycline in our case) is kept at a constant low concentration while the concentration of the other reactant (sensor RNA) is varied. To determine the binding affinity, the fraction of tetracycline that is in complex with the sensor RNA is plotted against the RNA concentration. The dissociation constant is the sensor RNA concentration that forces 50% of tetracycline to form complex with the sensor RNA (Fig. 9).
9.4 What is Fluorescence?
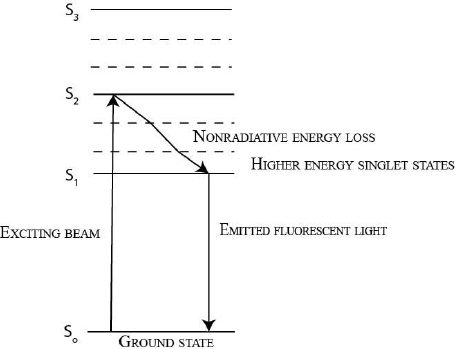
Fluorescence is a natural phenomenon. Some compounds, usually heterocyclic aromatic molecules, are able to absorb light of a specific energy and subsequently emit light that is lower in energy (larger wavelength) than the light absorbed. The wavelength of the light absorbed by the fluorescent compound is referred to as the excitation wavelength. The wavelength of the light emited by the fluorescent compound is referred to as the emission wavelength (Fig. 9.2).

During fluorescence, the incoming photons (excitation wave) excite electrons in the molecule to the higher energy excited states. The electrons lose some energy via nonradiative decay to reach the lowest energy excited state. From here, the excited electrons emit energy in form of photons (emission wave) to return to the ground state. The energy of the emission wave is lower than that of the excitation wave, because some energy was lost due to nonradiative decay, usually heat (Fig 9.3).
9.5 How Do We Measure Binding Affinity of the Tetracycline-Sensor RNA Complex?
To measure the binding affinity of the tetracycline-sensor RNA complex, you need to determine the fraction of tetracycline that is in complex with the toxin sensor RNA (fraction bound). To measure fraction bound you will take advantage of the natural fluorescence of tetracycline: once the sensor RNA forms a complex with tetracycline, the fluorescence of tetracycline decreases (quenching). The amount of quenching is proportional to the fraction of tetracycline that is bound to the sensor RNA (Fig 9.4).
To determine the dissociation constant (KD) you need to plot the amount of quenching (proportional to the fraction of tetracycline bound to the sensor RNA) against the sensor RNA concentration. This plot should shape as a hyperbola (saturation curve). Then you should fit the Equation 1 to determine the KD value (Fig. 9.5).
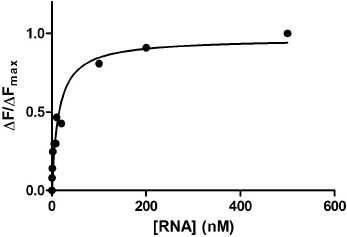

9.6 How do We Evaluate Binding Affinity?
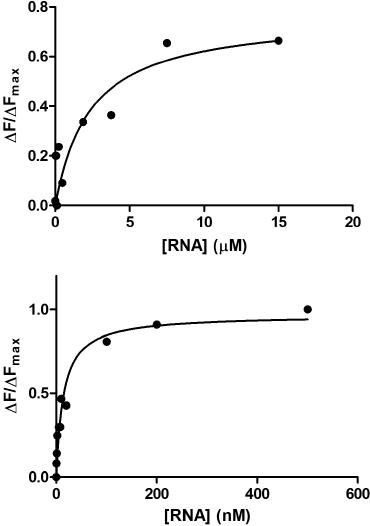
The goal of the mini-project was to identify parts of the toxin sensor that are essential for recognizing the antibiotic tetracycline. To reach the goal, you modified (mutated) evolutionary conserved parts of the toxin sensor and evaluated how well these mutated sensors were able to retain their ability to recognize tetracycline by measuring the KD of the mutant sensor RNA-tetracycline complex. If the mutated sensor retain its ability to recognize tetracycline (small KD value), the part of the sensor targeted for mutagenesis was not essential to recognize tetracycline. Likewise, if the mutated sensor lose its ability to recognize tetracycline (large KD value), the part of the sensor targeted for mutagenesis was important for tetracycline recognition (Fig 9.6).

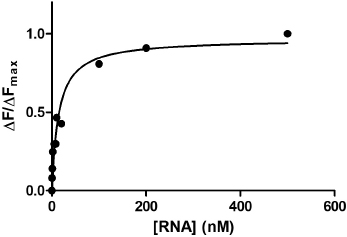
9.7 How do We Analyze Data?
Based on the data shown on Fig. 9.7, the mutant sensor-tetracycline complex has a KD value of about a 100 nM. Compared to a binding affinity (10 nM) reported for the wild-type sensor RNA tetracycline complex, the mutant sensor slightly lost its ability to recognize the antibiotic tetracycline. Based on these data, we conclude that the part of the toxin sensor mutated is likely to be important for tetracycline recognition. To determine the KD value of the mutant sensor-tetracycline complex more accurately, a higher mutant sensor RNA concentrations should be used in the future.
What are we doing today?
- Set up binding assays
- Use demo data to fit binding curve and determine KD
PROTOCOLS
Reagents and equipment needs are calculated per six student teams. There is ~20% excess included.
Equipment/glassware needed
- Micropipettes 20-100 μl and 2-20 μl
- Fluorescent plate reader
- 96-well plates
Solutions needed
- 5 x reaction buffer (100 mM Tris, pH=8.0; 500 mM KCl; 5 mM MgCl2)
- 20 nM tetracycline (in DMSO)
- Ribolock RNase inhibitor (Fermentas, 40 U/μl)
Binding assay setup
Each student should set up 2 assays per mutant and one assay using the wild type ykkCD RNA sensor.
1. Prepare 2 solutions for each row (assay)
RNA containing solution
20 μl 5x reaction buffer
10 μl 20 nM tetracycline
Up to 70 μl or 1 μM final concentration sensor RNA
0.5 μl Ribonuclease inhibitor
Water to 100 μl if needed
No RNA solution
120 μl 5x reaction buffer
60 μl 20 nM tetracycline
3 μl Ribonuclease inhibitor
417 μl water
2. In each row perform serial dilution using the solutions above as follows
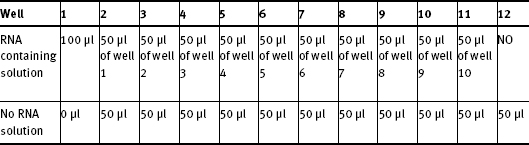
CALCULATE RNA CONCENTRATION OF EACH WELL ACCURATELY
4. Seal tray, cover with aluminum foil and incubate for 72 hrs. at 4 ºC.
Note to the instructor
The experiment in Chapter 9 was designed to determine the binding affinity of the mutant ykkCD sensor-tetracycline complex. The same protocol with minimal modifications can be used to measure binding affinity of any other fluorophore-macromolecule complex using fluorescent quenching. The binding assay worksheet may be incorporated into a lecture course that teaches binding affinity. 96-well plates used were manufactured by Corning Inc. (3991), but as long as the plates are black, flat bottom and have 96-wells, any other vendor might be used. Usage of RNase inhibitor is important to prevent RNA degradation especially considering the limited research experience of the experimenters. Assays may be read out by teaching assistants or by students that take the laboratory course at a different time of the week. A Tecan Infinite F200 plate reader was used to read out the assays; excitation wavelength was 380 nm, emission wavelength was 535 nm.
Analysis of Binding Experiments
In a common binding experiment to a set amount of one reagent (A) successive amounts of a second reagent (B) are added; the fractional amount bound (AB) is then measured. Since the second reagent (B) is added until all the first reagent (A) is bound, A is said to be “saturated” with B. Often the goal of the experiment is to determine the dissociation equilibrium constant, KD.
Whether we study a binding protein or RNA, etc., the binding equilibrium can be treated in the same way:

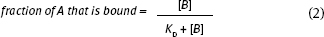
Equation 2 predicts a hyperbolic binding curve (see below). This is sometimes termed a “saturation” curve because A is finally saturated with B.
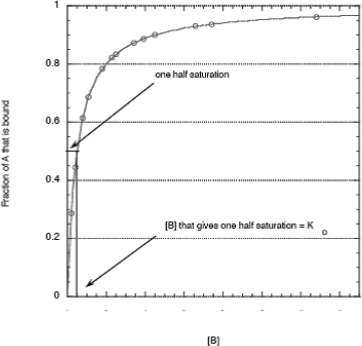
The dissociation equilibrium constant is equal to the concentration of B that gives one half-saturation. For the RNA sensor binding assay, you will use a set amount of tetracycline to which increasing amounts of RNA will be added. The fluorescence of tetracycline will decrease as it is bound by the RNA.
Fluorescent quenching is used to measure the fraction of tetracycline bound by RNA.
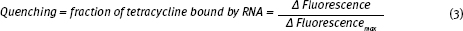
where

and
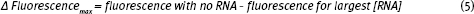

The RNA-Tetracycline Binding Worksheet contains problems that allow you to work through these calculations.
Once you have plotted the hyperbolic binding curve, you need to use a double reciprocal plot; this generally gives a better measure of KD. The reciprocal of the binding equation predicts a straight line plot:
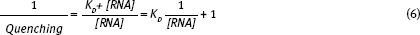
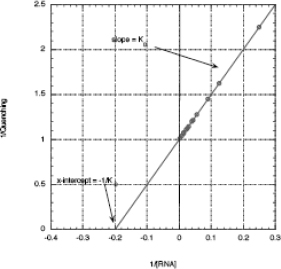
Binding Assays Prelab
Work with the following data from binding assays using two sensor RNA mutants. Please use a computer graphing program. Mutations are highlighted on the figure below.

1. (10 pts.) The fluorescence from tetracycline was measured as a function of [RNA] for Mutant #1 and the following results were obtained (Quenching = ΔFl/ΔFlmax):
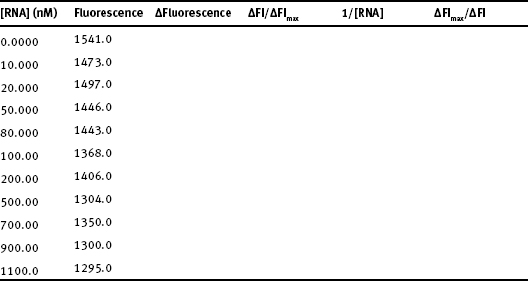
- a) Plot quenching versus [RNA] and estimate the KD for tetracycline-RNA binding. Show how you estimated the dissociation constant.
- b) Use a double reciprocal plot to get a better estimate of KD.
2. (15 pts.) Mutant #2 was also studied. The stock [RNA] was 2.4 × 10-5 M. The procedure for this experiment matched the one you are running in lab. Prepare a double reciprocal plot and determine the KD for mutant #2.

3. (5 pts.) Which mutation has a larger impact on tetracycline binding? Briefly explain.
Lab Report Outline and Point Distribution
1. Several sentences defining the goal/purpose of each procedure. Describe the role this step plays in the mini project (5 pts.).
2. Describe fluorescence (about five sentences; 5 pts.).
3. Show an example of your calculations for RNA concentration in the binding assay (3 pts.).
4. Use a computer to plot (20 pts. total):
- a. Quenching vs. [RNA] for each assay; determine the KD from the curves. Report error and standard deviation!
- b.
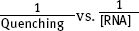 for each assay; determine the KD from these double reciprocal plots. Report error and standard deviation!
for each assay; determine the KD from these double reciprocal plots. Report error and standard deviation! - c. Do you consider your KD measurement reliable? Briefly explain. How would you modify your experiment to more accurately determine KD? For example, could you change the [RNA] range? Briefly explain (10 pts.).
5. In your judgment how did the mutation affect the ability of the sensor to recognize the antibiotic tetracycline? Do you think the mutated region was important for antibiotic recognition? How does the KD of the mutant sensor – tetracycline complex relate to that of the wild type sensor - tetracycline complex? (7 pts.).


