W2018_Bis2A_Lecture18_reading
- Page ID
- 10816
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Transport across the membrane
Design challenge problem and subproblems
General Problem: The cell membrane must simultaneously act as a barrier between "IN" and "OUT" and control specifically which substances enter and leave the cell and how quickly and efficiently they do so.
Subproblems: The chemical properties of molecules that must enter and leave the cell are highly variable. Some subproblems associated with this are: (a) Large and small molecules or collections of molecules must be able to pass across the membrane. (b) Both hydrophobic and hydrophilic substances must have access to transport. (c) Substances must be able to cross the membrane with and against concentration gradients. (d) Some molecules look very similar (e.g. Na+ and K+) but transport mechanisms must still be able to distinguish between them.
Energy story perspective
Transport across a membrane can be considered from an energy story perspective; it is a process after all. For instance, at the beginning of the process a generic substance X may be either on the inside or outside of the cell. At the end of the process, the substance will be on the opposite side from which it started.
e.g. X(in) ---> X(out),
where in and out refer to inside the cell and outside the cell, respectively.
At the beginning the matter in the system might be a very complicated collection of molecules inside and outside of the cell but with one molecule of X more inside the cell than out. At the end, there is one more molecule of X on the outside of the cell and one less on the inside. The energy in the system at the beginning is stored largely in the molecular structures and their motions and in electrical and chemical concentration imbalances across the cell membrane. The transport of X out of the cell will not change the energies of the molecular structures significantly but it will change the energy associated with the imbalance of concentration and or charge across the membrane. That is the transport will, like all other reactions, be either exergonic or endergonic. Finally, some mechanism or sets of mechanisms of transport will need to be described.
Energetics of transport
All substances that move through the membrane do so by one of two general methods, which are categorized based on whether or not the transport process is exergonic or endergonic. Passive transport is the exergonic movement of substances across the membrane. In contrast, active transport is the endergonic movement of substances across the membrane that is coupled to an exergonic reaction.
Passive transport
Passive transport does not require the cell to expend energy. In passive transport, substances move from an area of higher concentration to an area of lower concentration, down their concentration gradient. Depending on the chemical nature of the substance, different processes may be associated with passive transport.
Diffusion
Diffusion is a passive process of transport. A single substance tends to move from an area of high concentration to an area of low concentration until the concentration is equal across a space. You are familiar with diffusion of substances through the air. For example, think about someone opening a bottle of ammonia in a room filled with people. The ammonia gas is at its highest concentration in the bottle; its lowest concentration is at the edges of the room. The ammonia vapor will diffuse, or spread away, from the bottle; gradually, more and more people will smell the ammonia as it spreads. Materials move within the cell’s cytosol by diffusion, and certain materials move through the plasma membrane by diffusion.
Factors that affect diffusion
If unconstrained, molecules will move through and explore space randomly at a rate that depends on their size, their shape, their environment, and their thermal energy. This type of movement underlies the diffusive movement of molecules through whatever medium they are in. The absence of a concentration gradient does not mean that this movement will stop, just that there may be no net movement of the number of molecules from one area to another, a condition known as dynamic equilibrium.
Factors influencing diffusion include:
- Extent of the concentration gradient: The greater the difference in concentration, the more rapid the diffusion. The closer the distribution of the material gets to equilibrium, the slower the rate of diffusion becomes.
- Shape, size and mass of the molecules diffusing: Large and heavier molecules move more slowly; therefore, they diffuse more slowly. The reverse is typically true for smaller, lighter molecules.
- Temperature: Higher temperatures increase the energy and therefore the movement of the molecules, increasing the rate of diffusion. Lower temperatures decrease the energy of the molecules, thus decreasing the rate of diffusion.
- Solvent density: As the density of a solvent increases, the rate of diffusion decreases. The molecules slow down because they have a more difficult time getting through the denser medium. If the medium is less dense, rates of diffusion increase. Since cells primarily use diffusion to move materials within the cytoplasm, any increase in the cytoplasm’s density will decrease the rate at which materials move in the cytoplasm.
- Solubility: As discussed earlier, nonpolar or lipid-soluble materials pass through plasma membranes more easily than polar materials, allowing a faster rate of diffusion.
- Surface area and thickness of the plasma membrane: Increased surface area increases the rate of diffusion, whereas a thicker membrane reduces it.
- Distance traveled: The greater the distance that a substance must travel, the slower the rate of diffusion. This places an upper limitation on cell size. A large, spherical cell will die because nutrients or waste cannot reach or leave the center of the cell, respectively. Therefore, cells must either be small in size, as in the case of many prokaryotes, or be flattened, as with many single-celled eukaryotes.
Facilitated transport
In facilitated transport, also called facilitated diffusion, materials diffuse across the plasma membrane with the help of membrane proteins. A concentration gradient exists that allows these materials to diffuse into or out of the cell without expending cellular energy. In the case that the materials are ions or polar molecules (compounds that are repelled by the hydrophobic parts of the cell membrane), facilitated transport proteins help shield these materials from the repulsive force of the membrane, allowing them to diffuse into the cell.
Note: possible discussion
Compare and contrast passive diffusion and facilitated diffusion.
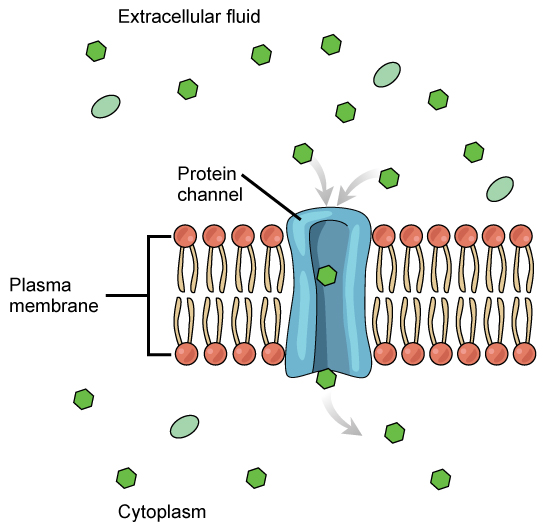
Channels
The integral proteins involved in facilitated transport are collectively referred to as transport proteins, and they function as either channels for the material or carriers. In both cases, they are transmembrane proteins. Different channel proteins have different transport properties. Some have evolved to have very high specificity for the substance that is being transported while others transport a variety of molecules sharing some common characteristic(s). The interior "passageway" of channel proteins have evolved to provide a low energetic barrier for transport of substances across the membrane through the complementary arrangement of amino acid functional groups (of both backbone and side-chains). Passage through the channel allows polar compounds to avoid the nonpolar central layer of the plasma membrane that would otherwise slow or prevent their entry into the cell. While at any one time significant amounts of water crosses the membrane both in and out, the rate of individual water molecule transport may not be fast enough to adapt to changing environmental conditions. For such cases Nature has evolved a special class of membrane proteins called aquaporins that allow water to pass through the membrane at a very high rate.
Channel proteins are either open at all times or they are “gated.” The latter controls the opening of the channel. Various mechanisms may be involved in the gating mechanism. For instance, the attachment of a specific ion or small molecule to the channel protein may trigger opening. Changes in local membrane "stress" or changes in voltage across the membrane may also be triggers to open or close a channel.
Different organisms and tissues in multicellular species express different sets of channel proteins in their membranes depending on the environments they live in or specialized function they play in an organisms. This provides each type of cell with a unique membrane permeability profile that is evolved to complement its "needs" (note the anthropomorphism). For example, in some tissues, sodium and chloride ions pass freely through open channels, whereas in other tissues a gate must be opened to allow passage. This occurs in the kidney, where both forms of channels are found in different parts of the renal tubules. Cells involved in the transmission of electrical impulses, such as nerve and muscle cells, have gated channels for sodium, potassium, and calcium in their membranes. Opening and closing of these channels changes the relative concentrations on opposing sides of the membrane of these ions, resulting a change in electrical potential across the membrane that lead to message propagation in the case of nerve cells or in muscle contraction in the case of muscle cells.
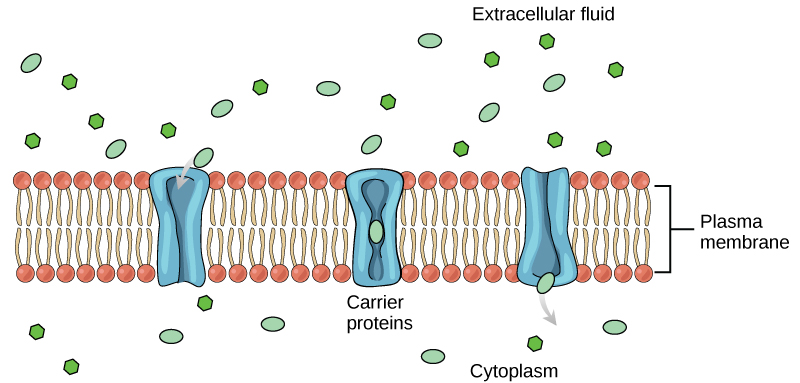
Carrier proteins
Another type of protein embedded in the plasma membrane is a carrier protein. This aptly named protein binds a substance and, in doing so, triggers a change of its own shape, moving the bound molecule from the outside of the cell to its interior; depending on the gradient, the material may move in the opposite direction. Carrier proteins are typically specific for a single substance. This selectivity adds to the overall selectivity of the plasma membrane. The molecular-scale mechanism of function for these proteins remains poorly understood.
Carrier protein play an important role in the function of kidneys. Glucose, water, salts, ions, and amino acids needed by the body are filtered in one part of the kidney. This filtrate, which includes glucose, is then reabsorbed in another part of the kidney with the help of carrier proteins. Because there are only a finite number of carrier proteins for glucose, if more glucose is present in the filtrate than the proteins can handle, the excess is not reabsorbed and it is excreted from the body in the urine. In a diabetic individual, this is described as “spilling glucose into the urine.” A different group of carrier proteins called glucose transport proteins, or GLUTs, are involved in transporting glucose and other hexose sugars through plasma membranes within the body.
Channel and carrier proteins transport material at different rates. Channel proteins transport much more quickly than do carrier proteins. Channel proteins facilitate diffusion at a rate of tens of millions of molecules per second, whereas carrier proteins work at a rate of a thousand to a million molecules per second.
Active transport
Active transport mechanisms require the use of the cell’s energy, usually in the form of adenosine triphosphate (ATP). If a substance must move into the cell against its concentration gradient—that is, if the concentration of the substance inside the cell is greater than its concentration in the extracellular fluid (and vice versa)—the cell must use energy to move the substance. Some active transport mechanisms move small-molecular weight materials, such as ions, through the membrane. Other mechanisms transport much larger molecules.
Moving against a gradient
To move substances against a concentration or electrochemical gradient, the cell must use energy. This energy is harvested from ATP generated through the cell’s metabolism. Active transport mechanisms, collectively called pumps, work against electrochemical gradients. Small substances constantly pass through plasma membranes. Active transport maintains concentrations of ions and other substances needed by living cells in the face of these passive movements. Much of a cell’s supply of metabolic energy may be spent maintaining these processes. (Most of a red blood cell’s metabolic energy is used to maintain the imbalance between exterior and interior sodium and potassium levels required by the cell.) Because active transport mechanisms depend on a cell’s metabolism for energy, they are sensitive to many metabolic poisons that interfere with the supply of ATP.
Two mechanisms exist for the transport of small-molecular weight material and small molecules. Primary active transport moves ions across a membrane and creates a difference in charge across that membrane, which is directly dependent on ATP. Secondary active transport describes the movement of material that is due to the electrochemical gradient established by primary active transport that does not directly require ATP.
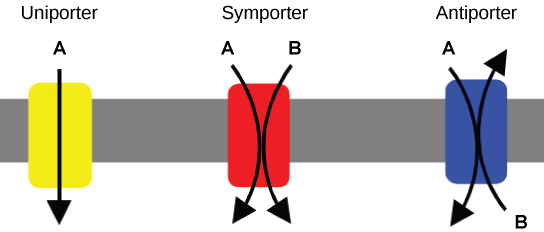
Carrier proteins for active transport
An important membrane adaption for active transport is the presence of specific carrier proteins or pumps to facilitate movement: there are three types of these proteins or transporters. A uniporter carries one specific ion or molecule. A symporter carries two different ions or molecules, both in the same direction. An antiporter also carries two different ions or molecules, but in different directions. All of these transporters can also transport small, uncharged organic molecules like glucose. These three types of carrier proteins are also found in facilitated diffusion, but they do not require ATP to work in that process. Some examples of pumps for active transport are Na+-K+ ATPase, which carries sodium and potassium ions, and H+-K+ ATPase, which carries hydrogen and potassium ions. Both of these are antiporter carrier proteins. Two other carrier proteins are Ca2+ATPase and H+ ATPase, which carry only calcium and only hydrogen ions, respectively. Both are pumps.
Primary active transport
In primary active transport, the energy is often - though not exclusively - derived directly from the hydrolysis of ATP. Often, primary active transport, such as that shown below, which functions to transport sodium and potassium ions allows secondary active transport to occur (discussed in the section below). The second transport method is still considered active because it depends on the use of energy from the primary transport.
One of the most important pumps in animal cells is the sodium-potassium pump (Na+-K+ ATPase), which maintains the electrochemical gradient (and the correct concentrations of Na+and K+) in living cells. The sodium-potassium pump moves K+ into the cell while moving Na+ out at the same time, at a ratio of three Na+ for every two K+ ions moved in. The Na+-K+ATPase exists in two forms depending on its orientation to the interior or exterior of the cell and its affinity for either sodium or potassium ions. The process consists of the following six steps.
- With the enzyme oriented towards the interior of the cell, the carrier has a high affinity for sodium ions. Three ions bind to the protein.
- ATP is hydrolyzed by the protein carrier and a low-energy phosphate group attaches to it.
- As a result, the carrier changes shape and re-orients itself towards the exterior of the membrane. The protein’s affinity for sodium decreases and the three sodium ions leave the carrier.
- The shape change increases the carrier’s affinity for potassium ions, and two such ions attach to the protein. Subsequently, the low-energy phosphate group detaches from the carrier.
- With the phosphate group removed and potassium ions attached, the carrier protein repositions itself towards the interior of the cell.
- The carrier protein, in its new configuration, has a decreased affinity for potassium, and the two ions are released into the cytoplasm. The protein now has a higher affinity for sodium ions, and the process starts again.
Several things have happened as a result of this process. At this point, there are more sodium ions outside of the cell than inside and more potassium ions inside than out. For every three ions of sodium that move out, two ions of potassium move in. This results in the interior being slightly more negative relative to the exterior. This difference in charge is important in creating the conditions necessary for the secondary process. The sodium-potassium pump is, therefore, an electrogenic pump (a pump that creates a charge imbalance), creating an electrical imbalance across the membrane and contributing to the membrane potential.
Link to learning
Visit the site to see a simulation of active transport in a sodium-potassium ATPase.
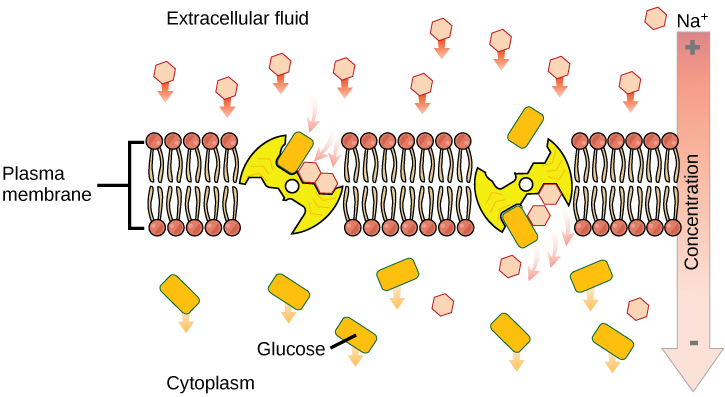
Secondary active transport (co-transport)
Secondary active transport brings sodium ions, and possibly other compounds, into the cell. As sodium ion concentrations build outside of the plasma membrane because of the action of the primary active transport process, an electrochemical gradient is created. If a channel protein exists and is open, the sodium ions will be pulled through the membrane. This movement is used to transport other substances that can attach themselves to the transport protein through the membrane. Many amino acids, as well as glucose, enter a cell this way. This secondary process is also used to store high energy hydrogen ions in the mitochondria of plant and animal cells for the production of ATP. The potential energy that accumulates in the stored hydrogen ions is translated into kinetic energy as the ions surge through the channel protein ATP synthase, and that energy is used to convert ADP into ATP.
The Cytoskeleton
The cytoskeleton is a network of different protein fibers that provides many functions: it maintains or changes the shape of the cell; it secures some organelles in specific positions; it enables movement of cytoplasm and vesicles within the cell; and it enables the cell to move in response to stimuli. There are three types of fibers within the cytoskeleton: microfilaments, intermediate filaments, and microtubules. Some of the cytoskeletal fibers work in conjunction with molecular motors which move along the fibers within the cell to carry out a diverse set of functions. There are two main families of cytoskeletally-associated molecular motors: dyneines and kinesins.
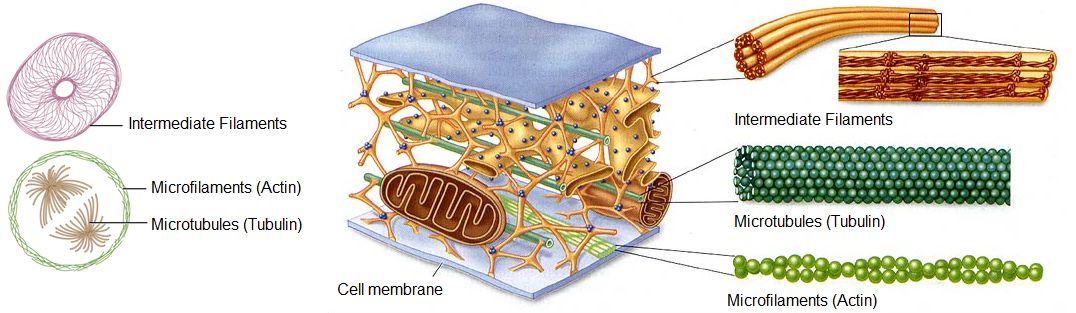
Figure 1. Microfilaments thicken the cortex around the inner edge of a cell; like rubber bands, they resist tension. Microtubules are found in the interior of the cell where they maintain cell shape by resisting compressive forces. Intermediate filaments are found throughout the cell and hold organelles in place.
Design challenge
Problem statement: Eukaryotic cells contain membrane-bound organelles that effectively separate materials, processes, and reactions from one another and from the cytoplasm. This in itself poses a problem for eukaryotes.
How can the cell purposely move and control the location of materials between these organelles? More specifically, how can a eukaryotic cell transport compounds from their place of origin (in most cases the cyotoplasm) to where they are needed (perhaps the nucleus, the mitochondria, or the cell surface)?
Note: possible discussion
Propose some reasons why cells—particularly large cells and/or cells with organelles—cannot rely on simple diffusion to move metabolites, building blocks, proteins, etc. to the locations in the cell where they are needed.
One possible solution is for the cell to create a network that can connect all the different parts of the cell together. This network could be used not only as a scaffold to hold components in place but also as a reference for direction. For example, we can use a map to determine the direction we need to travel and roads to connect and travel from home to campus. Likewise, an interconnecting network inside the cell can be used to direct and move compounds from one location to a final destination. Some of the required characteristics of this network are listed below. Can you add to this list?
Intracellular network
- The network needs to be extensive, and connect every area of the cell.
- The network needs to be flexible, able to change and adapt as the cell grows larger, divides into two cells, or physically moves from one environment to another.
- The network needs to be strong, able to hold up to mechanical pressure from inside the cell or from outside of the cell.
- The network needs to be composed of different fibers and each of these fibers needs to be for a specific connection in the cell. For example, certain fibers might be involved in holding organelles in place, and other fibers would be involved in connecting two different organelles.
- The fibers need to have directionality (or polarity), meaning they need to have a defined starting point and a defined end to help direct movement from one location to another.
- The fibers need to work with proteins that can convert chemical energy into kinetic energy, to actively transport compounds along the fibers.
Microfilaments
Actin
Microfilaments are cytoskeleton fibers composed of actin subunits. Actin is one of the most abundant proteins in eukaryotic cells and comprises 20% of total cellular protein by weight in muscle cells. The actin amino acid sequence is highly conserved in eukaryotic cells, meaning that the protein amino acid sequence, and therefore its final 3-D shape, has changed little over the course of evolution, maintaining more than 80% similarity between algae and humans.
Actin can be present as either a free monomer called G-actin (globular) or as part of a polymer microfilament called F-actin ("F" for filamentous). Actin must be bound to ATP in order to assemble into its filamentous form and maintain the structural integrity of the filament. The actin filament itself has structural polarity. This term "polarity", in reference to a cytoskeleton filament, does not mean what it did when we discussed polar functional groups earlier in this course. Polarity here refers to the fact that there are two distinct ends to the filament. These ends are called the "(-)" end and the "(+)" end. At the "(+)" end, actin subunits are adding onto the elongating filament and at the "(-)" end, actin subunits are disassembling or falling off of the filament. This process of assembly and disassembly is controlled by the ATP to ADP ratio in the cytoplasm.
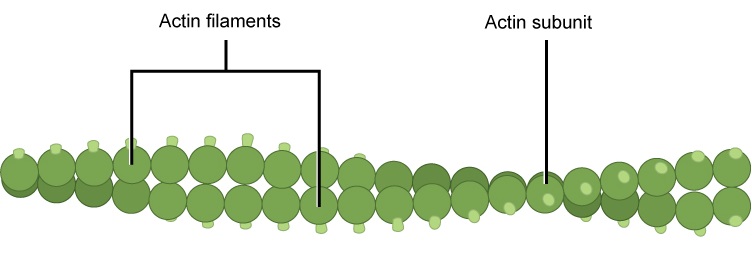
Figure 2. Microfilaments are the narrowest of the three cytoskeleton fibers, with a diameter of about seven nm. Microfilaments are composed of actin subunits which form into two intertwined strands.
Actin participates in many cellular processes, including muscle contraction, cell motility, cytokinesis during cell division, vesicle and organelle movement, and the maintenance of cell shape. Actin filaments serve as a track for the movement of a family of motor proteins called myosins discussed in more detail in a section below.
Link to learning:
To see an example of a white blood cell in action, click here and watch a short time-lapse video of the cell capturing two bacteria. It engulfs one and then moves on to the other.
Animations on actin filaments and how they work
Intermediate filaments
Intermediate filaments are made of several strands of fibrous proteins that are wound together. These elements of the cytoskeleton get their name from the fact that their diameter, eight to ten nm, is between those of the smaller microfilaments and the larger microtubules. The intermediate filaments are the most diverse group of cytoskeletal elements. Several types of fibrous proteins are found in the intermediate filaments. You are probably most familiar with keratin, the fibrous protein that strengthens your hair, nails, and the epidermis of the skin.
Figure 3. Intermediate filaments consist of several intertwined strands of fibrous proteins.
Intermediate filaments have no role in cell movement. Their function is purely structural. They bear tension, thus maintaining the shape of the cell, and anchor the nucleus and other organelles in place. The figure above shows how intermediate filaments create a cable-like supportive scaffolding inside the cell.
Microtubules
Microtubules are the largest component of the cytoskeleton and are found throughout the cytoplasm. These polymers are made up of globular protein subunits called α-tubulin and β-tubulin. Microtubules are found not only in eukaryotic cells but in some bacteria as well.
Both the α-tubulin and β-tubulin subunits bind to GTP. When bound to GTP, the formation of the microtubule can begin, this is called the nucleation event. As more GTP tubulin dimers assemble onto the filament, GTP is slowly hydrolyzed by β-tubulin to form GDP. Tubulin bound to GDP is less structurally robust and can lead to disassembly of the microtubule.
Much like the actin filaments discussed above, microtubules also have a distinct polarity that is critical for their biological function. Tubulin polymerizes end to end, with the β-subunits of one tubulin dimer contacting the α-subunits of the next dimer. These differences lead to different subunits being exposed on the two ends of the filament. The ends are designated the "(−)" and "(+)" ends. Unlike actin filaments, microtubules can elongate at both the "(+)" and "(-)" ends, but elongation is significantly more rapid at the "(+)" end.
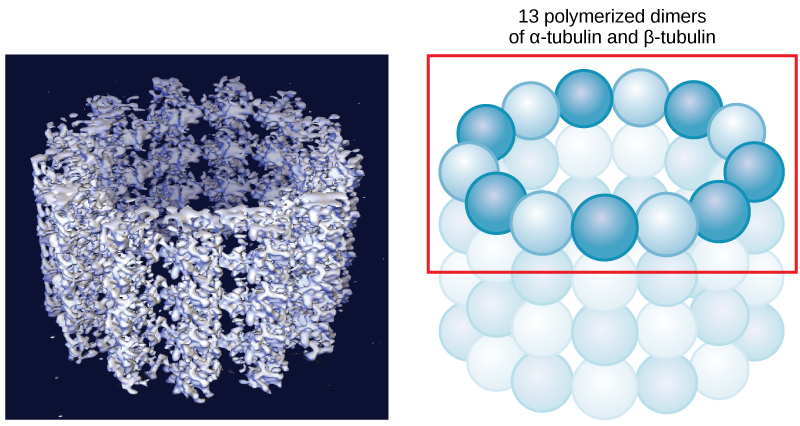
Figure 4. Microtubules are hollow. Their walls consist of 13 polymerized dimers of α-tubulin and β-tubulin (right image). The left image shows the molecular structure of the tube.
Microtubules help the cell resist compression, provide a track along which vesicles move through the cell, pull replicated chromosomes to opposite ends of a dividing cell, and are the structural elements of flagella, cilia, and centrioles (the latter are the two perpendicular bodies of the centrosome). In fact, in animal cells, the centrosome is the microtubule organizing center. In eukaryotic cells, flagella and cilia are quite different structurally from their counterparts in bacteria, discussed below.
Animations of the cytoskeleton
Where did these fibers come from?
The cytoskeleton probably has its origins in bacterial and/or archaeal ancestry. There are ancient relatives to both actin and tubulin in bacterial systems. In bacteria, the MreB protein and the ParM protein are believed to be early ancestors to Actin. MreB functions in maintaining cell shape and ParM functions in plasmid (DNA) partitioning. The FtsZ protein in bacteria functions in cytokinesis, it is a GTPase, spontaneously forms filaments and is hypothesized to be an ancient form of tubulin. These findings support the hypothesis that the eukaryotic cytoskeleton has its origins in the bacterial world.
Flagella and cilia
Flagella (singular=flagellum) are long, hair-like structures that extend from the plasma membrane and are used to move an entire cell (for example, sperm, Euglena). When present, the cell has just one flagellum or a few flagella. Cilia are short, hair-like structures that are used to move entire cells (such as paramecia) or substances along the outer surface of the cell (for example, the cilia of cells lining the fallopian tubes that move the ovum toward the uterus, or cilia lining the cells of the respiratory tract that trap particulate matter and move it toward your nostrils.) When cilia are present, there can be many of them, extending along the entire surface of the plasma membrane.
Despite their differences in length and number, flagella and cilia share a common structural arrangement of microtubules called a “9+2 array.” This is an appropriate name because a single flagellum or cilium is made of a ring of nine microtubule doublets, surrounding a single microtubule doublet in the center (Figure 5).
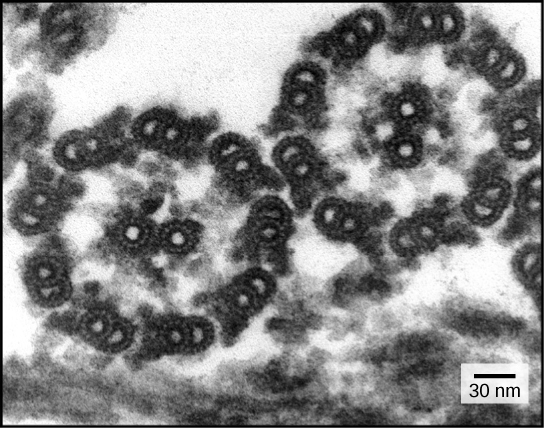
Figure 5. This transmission electron micrograph of two flagella shows the "9+2 array" of microtubules: nine microtubule doublets surround a single microtubule doublet. (credit: modification of work by Dartmouth Electron Microscope Facility, Dartmouth College; scale-bar data from Matt Russell)
For a video on flagellar and ciliar movement in eukaryotes, see the YouTube video: click here (you can skip the commericial).
Motor proteins
One function of the cytoskeleton is to move cellular components from one part of the cell to another. These cellular components are called "cargo" and are often stored within a vesicle for transport. You can think of the cytoskeleton as "railroad tracks" providing support and directionality inside of the cell.
Of course, if there are "railroad tracks" there needs to be an engine that can both move on the tracks and pull or push cargo along. In this case the engines are molecular motors that can move along the tracks in a specific direction. There are two families of molecular motors associated with the cytoskeleton; dyneines and kinesins. These motor proteins (train engines) and the cytoskeleton create a comprehensive network within the cell for moving vesicles (box cars) from one organelle to another or from one organelle to the cell surface.
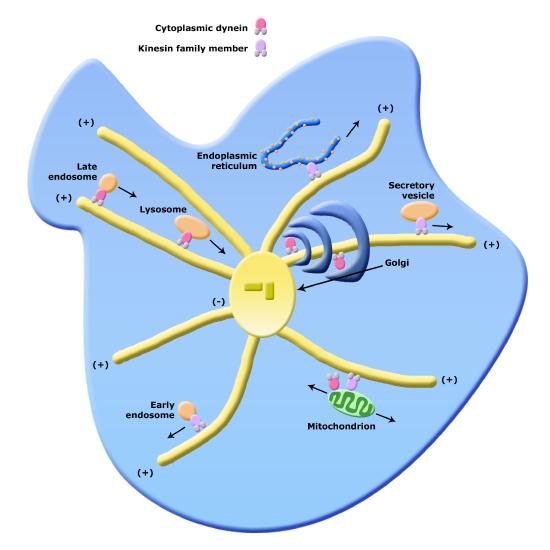
Figure 6. Organelle transport via microtubules and kinesins and dynes. Note that the figure is conceptual and only intended to show directionality of movement of various organelles; it does not necessarily represent all of their forms faithfully.
Cytoplasmic dyneins
Dynein is a protein complex that functions as a molecular motor. In cells, it converts the chemical energy from ATP hydrolysis into the mechanical energy of movement to 'walk' along the microtubule while carrying a vesicle. Dyneins bind to microtubules and move or "walk" from the plus "(+)" end of the cytoskeletal microtubule filament to the minus "(-)" end of the filament, which is usually oriented towards the cell center. Thus, they are often referred to as "minus end directed motors" and this vesicular transport is refereed to as retrograde transport. Cytoplasmic dynein moves processively along the microtubule, hydrolyzing ATP with each "step" it takes along the microtubule. During this process, one or the other of its "stalks" is always attached to the microtubule, allowing for the dynein motor (and its cargo) to "walk" a considerable distance along a microtubule without detaching.
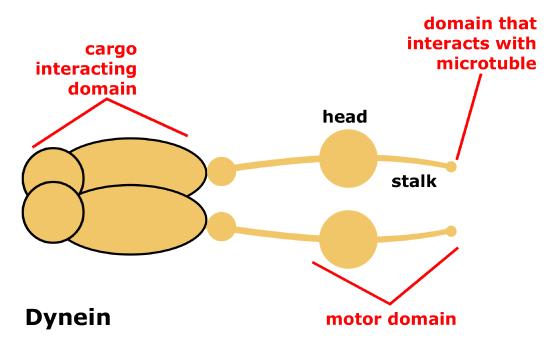
Figure 7. Schematic of cytoplasmic dynein motor protein. Dyneins are protein complexes composed of many smaller polypeptide subunits. The overall structure of the dynien motors are relatively simple, consisting of two identical complexes each having a motor domain that interacts with the microtubule, a stalk, or stem region that connects the motor head to the cargo interacting domain.
Cytoplamic dyneins are used in many different processes: they are involved in organelle movement such as the positioning of the Golgi complex and other organelles in the cell; they are used in the transport of cargo such as the movement of vesicles made by the endoplasmic reticulum, endosomes, and lysosomes; and they are responsible for the movement of chromosomes during cell division. Axonemal dyneins are motor proteins used in the sliding of microtubules in the axonemes of cilia and flagella in eukaryotic cells.
Kinesins
Kinesins, like cytoplasmic dyneins are motor-protein complexes that "walk" along the microtubules and are involved in vesicle transport. Unlike cytoplasmic dyneins, the polarity of kinesin movement is from the "(-)" end of the microtubule to the "(+)" end with the hydrolysis of ATP. In most cells, this entails transporting cargo from the center of the cell towards the periphery (the opposite direction to dyneins). This form of transport is known as anterograde or orthrograde transport. Like cytoplasmic dyneins, kinesins are involved in a variety of cellular processes including vesicle movement and chromosome movement during cell division.
The structure of kinesins are similar to cytoplasmic dyneins and is diagrammed in Figure 8. Members of the kinesin superfamily vary in shape, but the overall structure is that of a heterotetramer whose motor subunits (heavy chains) form a protein dimer (molecule pair) that binds two light chains.
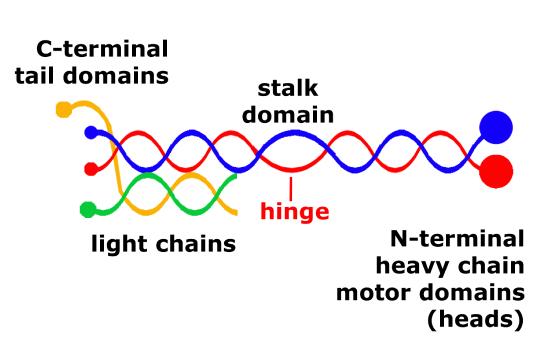
Figure 8. Schematic of kinesin motor proteins. The heavy chains comprise a globular head (the motor domain) at the amino terminal end connected via a short, flexible neck linker to the stalk—a long, central α-helical coiled-coil domain—that ends in a carboxy terminal tail domain which associates with the light-chains. The stalks of two light chains intertwine to form a coiled-coil that directs dimerization of the two heavy chains. In most cases transported cargo binds to the kinesin light chains, but in some cases cargo binds to the C-terminal domains of the heavy chains.
Animations of kinesin and dynein at work
How do the motors interact with cargo and the microtubules?
Cytoplasmic dyneins and kinesins interact with both cargo and microtubules in similar fashion. The light chains interact with receptors on the various cargo vesicles and the globular motor domains, specifically interact with the microtubules.
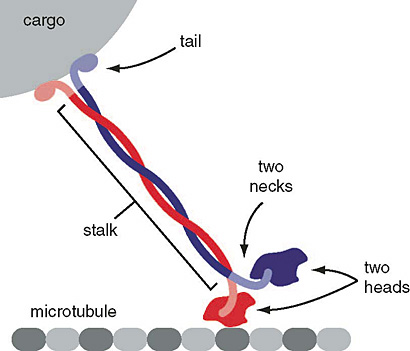
Figure 9. Schematic of kinesin motor protein carrying a cargo vesicle along a microtubule filament.
Note: possible discussion
What are the benefits for having multiple types of motor proteins? Multiple types of filaments? Filaments with polarity?