1.11: Glycolysis
- Page ID
- 8434
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Glycolysis: an overview
Organisms, whether unicellular or multicellular, need to find ways of getting at least two key things from their environment: (1) matter or raw materials for maintaining a cell and building new cells and (2) energy to help with the work of staying alive, growing, and reproducing (combating entropy). Energy and the raw materials may come from different places. For instance, photosynthetic organisms primarily harvest energy from sunlight and get raw materials for building biomolecules from non-organic sources like CO2. In contrast, some organisms rely on redox reactions with small molecules and/or reduced metals for energy and get their raw materials for building biomolecules from compounds unconnected to this chemical energy source. Meanwhile, some organisms (including ourselves), have evolved to get energy AND the raw materials for building and cellular maintenance from organic sources, for example, sugars supply both energy and carbon.
A metabolic pathway is a series of linked biochemical reactions. Glycolysis is the first metabolic pathway discussed in BIS2A. Because of its ubiquity in biology, it is hypothesized that glycolysis was probably one of the earliest metabolic pathways to evolve (more on this later). It is a 10-step pathway that is centered on the processing of glucose for both energy extraction from organic fuel and for the processing of the carbons in glucose into various other biomolecules (some of which are key precursors of many much more complicated biomolecules). Our study of glycolysis will be examined using the principles outlined in the design challenge and energy challenge that ask us to formally consider what happens to BOTH matter and energy in this multistep process.
The Energy Story and Design Challenge
Our investigation of glycolysis is a good opportunity to examine a biological processes using both the energy story and the design challenge perspectives.
The design challenge will try to get you to think actively about both broadly and specifically about why we are studying this pathway - what is so important about it? What "problems" does the evolution of a glycolytic pathway allow life to solve or overcome? We will also want to think about alternate ways to solve the same problems and why they may or may not have evolved. Later we will examine a hypothesis for how this pathway - and other linked pathways - may have actually evolved. Thinking about alternative strategies for satisfying various constraints will come in handy then.
In the context of the energy story, we will ask you to think about glycolysis as a process from which more general concepts can be learned by analyzing what happens to both matter and energy. Hopefully some general insights can be gained by carefully examining the process as a set of matter and energy inputs and outputs, a process with a beginning and an end. Although I do not want you to memorize all of these compounds and enzymes, we will focus in on a few of the steps as examples of more general concepts in the thermodynamics of how energy is harvested by the cells, and how cells can regulate metabolic flow.
So what is glycolysis? Let's start to find out. Please regard the discussion below as more than you need to know- consult the lectures for a more appropriate "overview". For example, if I do discuss a particular enzyme in an exam, I'll also tell you where it fits into glycolysis.

| Enzyme | Step | ΔG/(kJ/mol) | ΔG°'/(kJ/mol) |
|---|---|---|---|
| Hexokinase | 1 | -34 | -16.7 |
| Phosphoglucose isomerase | 2 | -2.9 | 1.67 |
| Phosphofructokinase | 3 | -19 | -14.2 |
| Fructose-bisphosphate aldolase | 4 | -0.23 | 23.9 |
| Triose phosphate isomerase | 5 | 2.4 | 7.56 |
| Glyceraldehyde 3-phosphate dehydrogenase | 6 | -1.29 | 6.30 |
| Phosphoglycerate kinase | 7 | 0.09 | -18.9 |
| Phosphoglycerate mutase | 8 | 0.83 | 4.4 |
| Enolase | 9 | 1.1 | 1.8 |
| Pyruvate kinase | 10 | -23.0 | -31.7 |
| The measurements of the energy at standard state (ΔG°'/(kJ/mol)) compared with measurements taken from a living cell (ΔG/(kJ/mol)). Reactions will occur in the direction that leads to a decrease in the value of the Gibbs free energy. Cellular measurements of ΔG can be dramatically different than ΔG°' measurements due to cellular conditions, such as concentrations of relevant metabolites etc. There are three large negative ΔG drops in the cell in the process of glycolysis. Due to this large negative ∆G, these reactions are considered "effectively irreversible" and are therefore often subject to regulation. | |||
The primary input into this pathway is a single molecule of glucose, though molecules may feed in and out of this pathway at various steps. We will focus our attention on (1) consequences of the overall process (2) several key reactions that highlight important types of biochemistry and physical chemistry principles we will want to carry forward to other contexts and (3) alternative fates of the intermediates and products of this pathway.
Note for reference that glycolysis is an anaerobic process, there is no requirement for molecular oxygen in glycolysis (oxygen gas is not a reactant in any of the chemical reactions in glycolysis). But the process can certainly occur in aerobic environments too (like our cells)- it is not strictly anaerobic. Glycolysis occurs in the cytosol or cytoplasm of cells. For a short (3 minute) overview YouTube video of glycolysis click here.
Design challenge:
You, as a cell, would like to generate some "ready cash" that you will use to fund the construction of expensive, complex molecules. This ready cash will be in the form of ATP. You are supplied with an external source of glucose, and an internal source of ADP and inorganic phosphate. Glucose has high potential energy- you know that breaking down and rearranging the bonds of glucose into lower potential energy molecules should provide you with the energy you need to construct one or more ATPs. But how are you going to couple the energy released/required by these two processes: the construction of ATP (endergonic) with the destruction of glucose (exergonic)?
First half of glycolysis: Energy Investment Phase
The first few steps of glycolysis are typically referred to as an "energy investment phase" of the pathway. This, however, doesn't make much intuitive sense (in the framework of a design challenge, it's not clear what problem this energy investment solves) if one only looks at glycolysis as an "energy producing" pathway and until these steps of glycolysis are put into a broader metabolic context. We'll try to build that story as we go, so for now just recall that we mentioned that some of the first steps are often associated with energy investment and ideas like "trapping" and "commitment" that are noted in the figure below.
Step 1 of glycolysis:
The first step in glycolysis shown below, is catalyzed by hexokinase (enzyme 1 in the figure below), an enzyme with broad specificity that catalyzes the phosphorylation of six-carbon sugars. Hexokinase catalyzes the phosphorylation of glucose, where glucose and ATP are substrates for the reaction, producing a molecule glucose-6-phosphate and ADP as products.
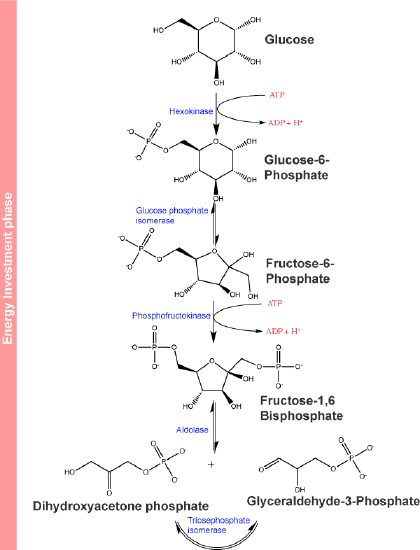
The first half of glycolysis is called the "energy investment" phase. In this phase, the cell expends two ATP into the reactions. Attribution: Marc T. Facciotti (original work)
Suggested discussion
The paragraph above states that the enzyme hexokinase has "broad specificity". This means that it can catalyze reactions with different sugars - not just glucose. From a molecular perspective, can you explain why this might be the case?
The conversion of glucose to the negatively charged glucose-6-phosphate significantly reduces the likelihood that the phosphorylated glucose leaves the cell by diffusion across the hydrophobic interior of the plasma membrane. It also "marks" the glucose in a way that effectively tags it for several different possible fates (see figure below).

Note that this figure indicates that glucose-6-phosphate can, depending on cellular conditions, be directed to multiple fates. While it is a component of the glycolytic pathway it is not only involved in glycolysis but also in the storage of energy as glycogen and in the building of various other molecules like nucleotides.
Source: Marc T. Facciotti (original work)
As the figure above indicates, glycolysis is but one possible fate for glucose-6-phosphate (G6P). Depending on cellular conditions, G6P may be diverted to the biosynthesis of glycogen (an form of energy storage) or it may be diverted into the pentose phosphate pathway for the biosynthesis of various biomolecules, including nucleotides. This means that G6P, while involved in the glycolytic pathway, is not solely tagged for oxidation at this phase. Hopefully, showing the broader context that this molecule is involved in (in addition to the rationale that tagging glucose with a phosphate decreases the likelihood that it will leave the cell) helps to explain the seemingly contradictory (if you only consider glycolysis as an "energy producing" process) reason for transferring energy from ATP onto glucose if it is only to be oxidized later - that is, glucose is not only used by the cell for harvesting energy and several other metabolic pathways that depend on the transfer of the phosphate group. But we'll also see that we are beginning to create a molecule that will be a powerful phosphate donor- powerful enough to push a phosphate onto an ADP, to make an ATP. Addition of a another phosphate to this organic compound later in glycolysis sourced from inorganic phosphate (free-floating, not nucleotide-bound phosphate) will make this investment worthwhile, in terms of net production, rather then hydrolysis, of ATP.
Step 2 of glycolysis:
In the second step of glycolysis, an isomerase catalyzes the conversion of glucose-6-phosphate into one of its isomers, fructose-6-phosphate. An isomerase is an enzyme that catalyzes the conversion of a molecule into one of its isomers.
Step 3 of glycolysis:
The third step of glycolysis is the phosphorylation of fructose-6-phosphate, catalyzed by the enzyme phosphofructokinase. A second ATP molecule donates a phosphate to fructose-6-phosphate, producing fructose-1,6-bisphosphate and ADP as products. In this pathway, phosphofructokinase is a rate-limiting enzyme and its activity is tightly regulated. It is allosterically activated by AMP when the concentration of AMP is high and moderately allosterically inhibited by ATP at the same site. Citrate - a compound we'll discuss soon - also acts as a negative allosteric regulator of this enzyme (allostery will be further discussed when we study protein structure- for now, suffice it to say that the enzyme's function is regulated by the concentration of AMP, or citrate, respectively). In this way, phosphofructokinase monitors or senses molecular indicators of the energy status of the cells and can in response act as a switch that turns on or off the flow of substrate through the rest of the metabolic pathway depending on whether there is “sufficient” ATP available in the system. The conversion of fructose-6-phosphate into fructose 1,6-bisphosphate is sometimes referred to as a "commitment" step by the cell to the oxidation of the molecule in the rest of the glycolytic pathway by creating a substrate for and helping to energetically drive the next highly endergonic (under standard conditions) step of the pathway.
Step 4 of glycolysis:
In the fourth step in glycolysis the enzyme Fructose-bisphosphate aldolase cleaves 1,6-bisphosphate into two three-carbon isomers: dihydroxyacetone-phosphate and glyceraldehyde-3-phosphate.
Step 5 of glycolysis:
An isomerase transforms the dihydroxyacetone-phosphate into its isomer, glyceraldehyde-3-phosphate. The 6 carbon glucose has therefore now been converted into two phosphorylated 3-carbon molecules of G3P.
Second Half: Energy Payoff Phase
If viewed in the absence of other metabolic pathways, glycolysis has thus far cost the cell two ATP molecules and produced two small, three-carbon sugar molecules (G3P). So far, not so good in terms of our overall challenge- get build ATP by breaking down glucose. When viewed in a broader context this investment of energy to produce a variety of molecules that can be used in a variety of other pathways and doesn't seem like such a bad investment, and as we will see below, the rest of the glycolytic pathway will result in a net profit- more ATPs will be built than are destroyed.
Our two molecules of G3P can proceed through the second half of glycolysis. We now examine these reactions.
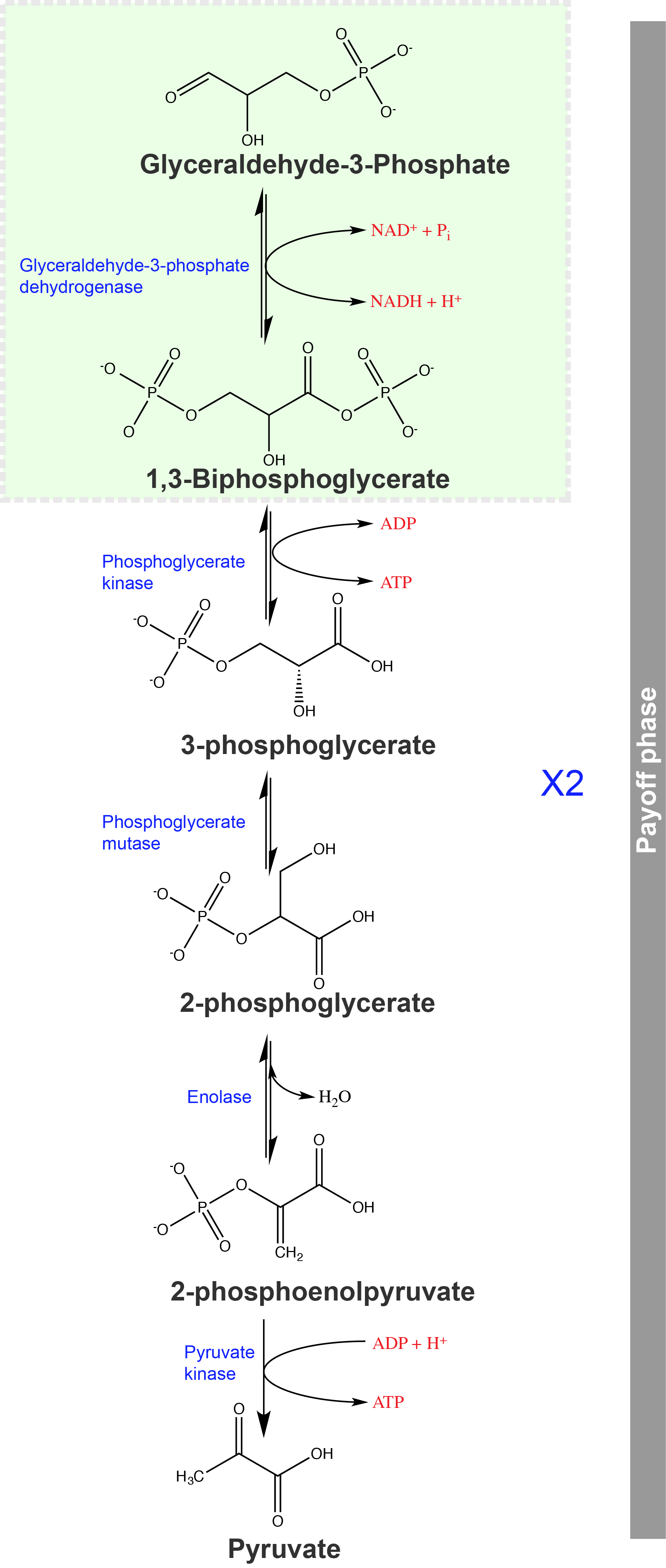
The second half of glycolysis is called the energy payoff phase. In this phase, the cell gains two ATP (from internal ADP + Pi) and 2 NADH (from internal NAD+) molecules. At the end of this phase glucose has become partially oxidized to form pyruvate.
Attribution: Marc T. Facciotti (original work).
Step 6 of glycolysis:
The sixth step is key and one from which we can now leverage our understanding of the several types of chemical reactions. If you're energy-focused, this is finally a step of glycolysis where some of the sugar is oxidized. The reaction is catalyzed by the enzyme glyceraldehyde-3-phosphate dehydrogenase. This enzyme catalyzes a multistep reaction between three substrates, glyceraldehyde-3-phosphate, the cofactor NAD+, and inorganic phosphate (Pi) and produces three products 1,3-bisphosphoglycerate, NADH and H+. One can think of this reaction as two reactions: (1) an oxidation/reduction and (2) a condensation reaction in which an inorganic phosphate is transferred onto a molecule. In this particular case, the redox reaction (a transfer of electrons off of G3P and onto NAD+) is exergonic and the phosphate transfer happens to be endergonic. The net standard free energy change- adding these two reactions together- hovers around zero. The enzyme here acts as a molecular coupling agent to couple the energetics of the exergonic reaction to that of the endergonic reaction; the ∆G of the oxidation of G3P drives the condensation of Pi onto the molecule. This process happens through a multi-step mechanism in the enzyme's active site and involves the chemical activity of a variety of functional groups. Neither of these reactions can occur (under these conditions) in the absence of the enzyme- the activation energy is too high. The trick the enzyme performs here is the physical coupling of the catalyzed exergonic reaction to the simultaneously catalyzed endergonic reaction. The enzyme thereby "allows" a favorable reaction to occur, but only when a normally unfavorable reaction occurs simultaneously. This coupling of favorable and unfavorable reactions is an essential concept in how life (superficially) appears to defeat the 2nd Law.
It is important to note that this reaction depends upon the availability of the oxidized form of the electron carrier, NAD+. If we consider that there is a limiting pool of NAD+ we can then conclude that the reduced form of the carrier (NADH) must be continuously oxidized back into NAD+ in order to keep this step going. If NAD+ is not available, the second half of glycolysis slows down or stops. It also depends on the availability of ADP- but of course, that's the point of the pathway- to change ADP into energy-rich ATP.
Step 7 of glycolysis:
The seventh step of glycolysis, catalyzed by phosphoglycerate kinase (an enzyme named for the reverse reaction), 1,3-bisphosphoglycerate transfers a phosphate to ADP, forming one molecule of ATP and a molecule of 3-phosphoglycerate. This reaction is exergonic and is also an example of the formation of ATP via "substrate-level phosphorylation"- as opposed to "oxidative phosphorylation", which we'll discuss in another reading. In substrate level phosphoryation, the phosphate is donated by a high-energy carbon compound (essentially a compound that is a stronger phosphate donor than ATP). In oxidiative phophorylation, in contrast, we'll build ATP from ADP and Pi, and power that using a drop in the concentration of protons.

Note: Possible discussion
If a transfer of a phosphate from 1,3-BPG to ADP is exergonic, what does that say about the free energy of hydrolysis of the phosphate from 1,3-BPG as compared to the free energy of hydrolysis of the terminal phosphate on ATP?
Step 8 of glycolysis:
In the eighth step, the remaining phosphate group in 3-phosphoglycerate moves from the third carbon to the second carbon, producing 2-phosphoglycerate (an isomer of 3-phosphoglycerate). The enzyme catalyzing this step is a mutase (a kind of isomerase).
Step 9 of glycolysis:
Enolase catalyzes the ninth step. This enzyme causes 2-phosphoglycerate to lose water from its structure; this is a dehydration reaction, resulting in the formation of a double bond that increases the potential energy in the remaining phosphate bond and produces phosphoenolpyruvate (PEP).
Step 10 of glycolysis:
The last step in glycolysis is catalyzed by the enzyme pyruvate kinase. Conversion of the serendipitously named PEP into pyruvate results in the production of a second ATP molecule by substrate-level phosphorylation and the compound pyruvic acid (or its salt form, pyruvate). The enzyme in this case is named for the reverse reaction of pyruvate’s conversion into PEP (kinases take perform the general reaction R + ATP --> ADP + R-P). Many enzymes in enzymatic pathways are named for the reverse reactions, since the enzymes always can catalyze both forward and reverse reactions (these may have been described and discovered initially by assay of the reverse reaction that takes place in vitro).
Outcomes of Glycolysis
A couple of things to consider:
One of the clear outcomes of glycolysis is the biosynthesis of compounds that can enter into a variety of metabolic pathways. Likewise compounds coming from other metabolic pathways can feed into glycolysis at various points. So, this pathway can be part of a central exchange for carbon flux within the cell.
If glycolysis is run long enough, the constant oxidation of glucose with NAD+ can leave the cell with a problem; how to regenerate NAD+ from the 2 molecules of NADH produced. If the NAD+ is not regenerated all of the cell's NAD will be nearly completely transformed into NADH. So how do cells regenerate NAD+ ? This is another design challenge.
Pyruvate is not completely oxidized, there is still some energy to be extracted - how might this happen? Also, what should the cell do with all of that NADH? Is there any energy there to extract?
Note: Strongly suggested discussion/exercise
Can you write an energy story for the overall process of glycolysis. For energy terms, just worry about describing things in terms of whether they are exergonic or endergonic. When I say overall process I mean overall process: glucose should be listed in the reactants and pyruvate listed on the product side of the arrow- you can skip the intermediates. You are of course welcome to do this in a more detailed fashion too.

