2.2: Introduction to Bacterial Growth and Aseptic Techniques
- Page ID
- 52337
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Outcomes
- Describe general characteristics of bacterial growth on agar plates
- Explain how to inoculate growth media using proper aseptic procedures
- Describe the process for inoculating sterile media
- Describe the procedure (T-streak) for isolation of single bacterial colonies
Characteristics of Bacterial Growth 
Even on general purpose growth media, bacteria can exhibit characteristic growth patterns. On agar plates, bacteria grow in collections of cells called colonies. Each colony arises from a single bacterium or a few bacteria. Although individual cells are too small to be viewed, masses of cells can be observed. Colonies can have different forms, margins, elevations, and colors. Observing colony characteristics is one piece of information that microbiologists can use to identify unknown bacteria. Shown below are isolated colonies of S. aureus on a blood agar plate. Colonies that are visible to the human naked eye contains tens of thousands or even millions of individual bacteria!!
Image 1: Notice individually isolated colonies: Large, creamy white, circular, beta-hemolytic colonies typical of Staphylococcus aureus cultured on Blood agar. Image by Rebecca Buxton, University of Utah, Salt Lake City, UT.
Colony morphology can be an aid in the identification of microorganisms.
Although colony morphology cannot be employed as the sole identifying criterion, it is a useful trait in the classification of many common types of microorganisms. Six parameters are normally used to describe microbial colonies growing on an agar surface:
a. Size: pinpoint, small, medium, or large; range: < l mm - 3cm
b. Color: absolutely white, various degrees of pigmentation
c. Texture: the texture of the colony as determined by touching the colony
with a needle; smooth (buttery), dry (granular), or mucoid (slimy) and the
appearance as judged by the manner in which the colony refracts light;
clear, glistening, dense, opaque, or translucent.
d. Form: the shape of the colony; circular, irregular, filamentous, or rhizoid
e. Elevation: the degree to which colony growth is raised; flat, raised,
convex or umbonate
f. Margin: the shape of the edge or margin of the colony
Figure 1: Different colony morphologies/characteristics
Image 2: Example of circular formed colonies--Serratia marcescens colonies cultivated on trypticase soy agar. Image by Bryan MacDonald, Christopher Adams, and Kyle Smith, Brigham Young University, Provo, UT.
Image 3: Undulate form colonies-- Streak plate isolation of Mycobacterium smegmatis on trypticase soy agar (TSA) incubated for 96 hours at 37oC. Note the rough texture of colonies characteristic of this organism. Image by Tasha L. Sturm, Cabrillo College, Aptos, CA.
Image 4: Irregular form colonies-- Mycobacterium marinum cultivated on Mycobacterium 7H11 agar with oleic acid-albumin-dextrose-catalase enrichment. Image by Richard A. Robison, Gable Moffitt, Neal Thomson, and Marissa Cohen, Brigham Young University, Provo, UT.
Aseptic Technique and Inoculation
In nature, microorganisms usually exist as mixed populations of different species of bacteria, fungi, and even viruses. If we are to study, characterize, and identify microorganisms, we must have the organisms in the form of a pure culture, that is of only one species of microorganism. A pure culture is one in which all organisms are descendants of the same organism. In working with microorganisms we must also have a sterile nutrient-containing-medium in which to grow the organisms. Anything in or on which we grow a microorganism is termed a medium. A sterile medium is one which is free of all life forms. It is usually sterilized by heating it to a temperature at which all contaminating microorganisms are destroyed. Finally, in working with microorganisms, we must have a method of transferring growing organisms (called the inoculum) from a pure culture to a sterile medium without introducing any unwanted outside contaminants. This method of preventing unwanted microorganisms from gaining access is termed aseptic technique. (1)
Inoculation is the purposeful introduction of bacteria into a sterile growth medium. A material is sterile when it has no living organisms present; contamination is the presence of unwanted microorganisms. Aseptic techniques are practices that prevent the contamination of growth media.
When working in a microbiology laboratory, you must always remember that bacteria are present on all surfaces in the lab, as well as on your own hands and clothing. Aseptic techniques are designed to prevent the transfer of bacteria from the surrounding environment into a culture medium. These techniques require care and concentration. Pay attention to what you are doing at all times!
Aseptic techniques include the following practices:
1. Minimize the time that cultures and growth media are open to the environment.
2. Disinfect the work area before and after use.
3. Do not touch or breathe into the sterile culture media or the stock cultures.
4. Loops, needles, pipets, etc. should be sterilized before they are used.
5. When working with tubes, the tube caps should not be placed on the table top; they should be held in your hand while inoculating.
6. When removing the caps from test tubes, heat/flame the lip of the test tube after the cap is removed. This heats the air inside the tube, so the air moves out of the tube, preventing contaminants from entering the tube.
7. Information about the use of the bacticinerator can be found in the Procedures page and view videos 2 and 3 below.
Watch this video 1: Aseptic Technique Tips
Watch Video 1: Aseptic Techniques tips. Video by Dr. Gary Kaiser (CCBC). (5:56) URL: https://youtu.be/_tMM0F0Pr60
General Procedure for inoculating media
1. Sterilize an inoculating loop or needle in the flame of a Bunsen burner. The portion of the loop or needle that will contact the stock culture or the growth medium must turn bright orange for effective sterilization. For the most rapid sterilization, place the loop at the top of the inner blue cone of flame—this is where the temperature of the Bunsen burner is the hottest. Or if it is a bacticinerator, make sure the loop is in the body of the bacticinerator and heat for 10 sec. Remove the loop from the flame after it is properly heated- keeping the loops in the flame for too long will eventually cause them to crack.
2. If you are picking a colony from a plate, cool the inoculating loop on agar that does not contain any bacterial colonies.
3. Pick a small amount of bacteria (you do not need much). If you are inoculating a tube of broth or an agar slant, remove the cap of the tube (do not set the cap down on the table) and flame the lip of the tube. Throughout the procedure, hold the tube at an angle to reduce the probability of particles entering the opening. Insert the loop into the tube and transfer bacteria to the growth medium. Be careful that only the sterilized part of the loop touches the tube or enters the growth medium.
4. Flame the lip of the test tube before replacing the cap.
5. Sterilize the inoculating loop again.
Watch this video 2: Aseptic Transfer
Watch Video 2: Aseptic transfers: a variety of transfers from solid to liquid. This video was filmed in the Microbiology teaching labs at NC State. (7:39) URL: https://youtu.be/sFbJmJeTIFc
Streaking for single colonies: T-streak
In the real world outside the laboratory, bacteria grow in communities made of many bacterial species. If you need to identify the types of bacteria present in environmental or medical samples, you must have a way to separate out the different types and produce pure cultures. A pure culture contains a single bacterial species, whereas a mixed culture may contain many different types of bacteria. The T-streak method describes the method that you will use to separate different types of bacteria in a mixture.
To maintain a pure culture or to obtain a pure culture of a microorganism, a quadrant/T/isolation/streak plate is performed. The T-streak is a form of dilutions on a solid surface (Franklund, 2018). In this technique, the plate is divided into sections. Bacteria are deposited in the first section at full strength from the original source. Then the inoculating loop is sterilized. From this point on, no additional cells are added to the agar surface. The sterile loop is used to spread out cells that have already been placedon the plate. After spreading cells from the first area to the second, the loop is sterilized again. This eliminates extra cells from the loop. The sterile loop is used to spread some cells from the second area into the third area diluting them further. (In a quadrant streak, cells are spread into a forth area as described.) After all the regions have been inoculated, the hope is that in the last section cells are far enough apart so that they grow up into isolated colonies. This technique allows one to observe isolated colonies and characterize them and determine if your observations are consistent with our expectations for the organism you are working with. If you are working with a pure culture, you would expect that all the colonies would look the same, similar size, color, shape etc. One or more different looking colonies indicates your culture was contaminated or you created contamination by poor aseptic technique.
Recall that each individual colony that you see on an agar plate represents billions of bacteria that originated from a single cell, and therefore should be clones of each other. If you want a pure culture then, there should only be 1 species or strain of that bacterium on that plate. We typically do a T-streak in the lab where you draw a large “T” on the back of the agar plate to divide it into 3 sectors. Watch video 3 below for a demonstration.
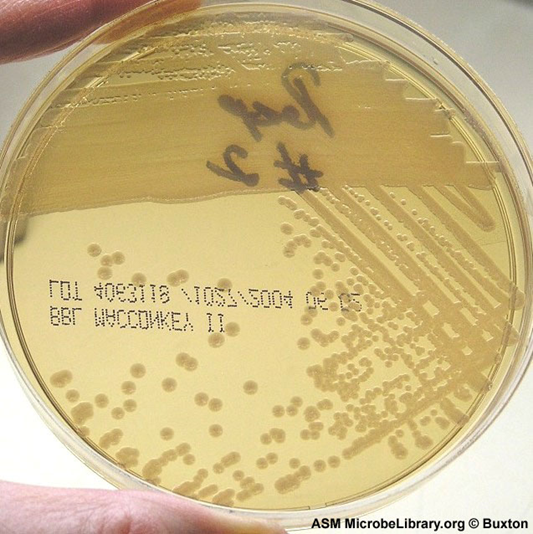
Image 5: Agar plate with T-streak of E. coli . This is a pure culture and notice the isolated colonies in the last sector of the plate, they appear as single, individual, round colonies. Image by Rebecca Buxton, University of Utah, Salt Lake City, UT.
Watch video 3: Isolating bacterial colonies using a T-streak method
Watch video 3: Isolating bacterial colonies using a T-streak. Excellent summary video of many things we talked about in this section: the difference between a cell vs a colony, how to separate bacterial species using a T-streak isolation method, common mistakes when doing a T-streak, and the results of a T-streak. They use disposable loops here and sterilize the loop with 70% ethanol between each section when doing the T-streak. You can also discard the loop (in biohazard) when streaking between sections, or use a metal loop (usually made of nickel-chrome wire) that you sterilize using a bunsen burner or a bacticinerator. Video by ID laboratory videos (9:17) URL: https://youtu.be/c1onYow0O58
Watch Video 4: Doing a T-streak at NC State
Watch video 4: How to perform a T-streak for isolated colonies. This video was filmed in the Microbiology laboratories at NC State. It will familiarize you with the use of a bacticinerator for sterilizing a metal inoculating loop. (7:39) URL:https://youtu.be/NsQv7QOmdXo
Notes about Labeling and Incubating Plates
1. Always label your plates/tubes BEFORE you do your inoculations. You can use Sharpies on the plates, but wax markers ONLY on tubes. When labeling tubes, label the tube itself—don’t label the cap!
2. Make sure you label the bottom of the plates (the part of the plate that holds the agar).
3. Place plates inverted (upside down) for incubation. This prevents condensation from falling on the surface of the agar and disrupting the streaking pattern.
Contributions and Attributions
1) Dr. Gary Kaiser (COMMUNITY COLLEGE OF BALTIMORE COUNTY, CATONSVILLE CAMPUS)
2) 2.1: Introduction by Joan Petersen & Susan McLaughlin, is licensed CC BY-NC-SA

