10: Environmental Factors
- Page ID
- 10674
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Competition is fierce out in the microbial world (non-microbial world, too!) and resources can be scarce. For those microbes that are willing and able to adapt to what might be considered a harsh environment, it can certainly mean less competition. So what environmental conditions can affect microbial growth? Temperature, oxygen, pH, water activity, pressure, radiation, lack of nutrients…these are the primary ones. We will cover more about metabolism (i.e. what type of food can they eat?) later, so let us focus now on the physical characteristics of the environment and the adaptations of microbes.
Osmolarity
Cells are subject to changes in osmotic pressure, due to the fact that the plasma membrane is freely permeable to water (a process known as passive diffusion). Water will generally move in the direction necessary to try and equilibrate the cell’s solute concentration to the solute concentration of the surrounding environment. If the solute concentration of the environment is lower than the solute concentration found inside the cell, the environment is said to be hypotonic. In this situation water will pass into the cell, causing the cell to swell and increasing internal pressure. If the situation is not rectified then the cell will eventually burst from lysis of the plasma membrane. Conversely, if the solute concentration of the environment is higher than the solute concentration found inside the cell, the environment is said to be hypertonic. In this situation water will leave the cell, causing the cell to dehydrate. Extended periods of dehydration will cause permanent damage to the plasma membrane.

Hypertonic vs. Hypotonic Solutions.
Cells in a hypotonic solution need to reduce the osmotic concentration of their cytoplasm. Sometimes cells can use inclusions to chemically change their solutes, reducing molarity. In a real pinch they can utilize what are known as mechanosensitive (MS) channels, located in their plasma membrane. MS channels open as the plasma membrane stretches due to the increased pressure, allowing solutes to leave the cell and thus lowering the osmotic pressure.
Cells in a hypertonic solution needing to increase the osmotic concentration of their cytoplasm can take up additional solutes from the environment. However, cells have to be careful about what solutes they take up, since some solutes can interfere with cellular function and metabolism. Cells need to take up compatible solutes, such as sugars or amino acids, which typically will not interfere with cellular processes.
There are some microbes that have evolved to extreme hypertonic environments, specifically high salt concentrations, to the point where they now require the presence of high levels of sodium chloride to grow. Halophiles, which require a NaCl concentration above 0.2 M, take in both potassium and chloride ions as a way to offset the effects of the hypertonic environment that they live in. Their evolution has been so complete that their cellular components (ribosomes, enzymes, transport proteins, cell wall, plasma membrane) now require the presence of high concentrations of both potassium and chloride to function.
pH
pH is defined as the negative logarithm of the hydrogen ion concentration of a solution, expressed in molarity. The pH scale ranges from 0 to 14, with 0 representing an extremely acidic solution (1.0 M H+) and 14 representing an extremely alkaline solution (1.0 x 10-14 M H+). Each pH units represents a tenfold change in hydrogen ion concentration, meaning a solution with a pH of 3 is 10x more acidic than a solution with a pH of 4.
Typically cells would prefer a pH that is similar to their internal environment, with cytoplasm having a pH of 7.2. That means that most microbes are neutrophiles (“neutral lovers”), preferring a pH in the range of 5.5 to 8.0. There are some microbes, however, that have evolved to live in the extreme pH environments.
Acidophiles (“acid lovers”), preferring an environmental pH range of 0 to 5.5, must use a variety of mechanisms to maintain their internal pH in an acceptable range and preserve the stability of their plasma membrane. These organisms transport cations (such as potassium ions) into the cell, thus decreasing H+ movement into the cell. They also utilize proton pumps that actively pump H+ out.
Alkaliphiles (“alkaline lovers”), preferring an environmental pH range of 8.0 to 11.5, must pump protons in, in order to maintain the pH of their cytoplasm. They typically employ antiporters, which pump protons in and sodium ions out.

pH Scale. OpenStax, Inorganic Compounds Essential to Human Functioning. OpenStax CNX. Jun 18, 2013 http://cnx.org/contents/e4e45509-bfc0-4aee-b73e-17b7582bf7e1@3.
Temperature
Microbes have no way to regulate their internal temperature so they must evolve adaptations for the environment they would like to live in. Changes in temperature have the biggest effect on enzymes and their activity, with an optimal temperature that leads to the fastest metabolism and resulting growth rate. Temperatures below optimal will lead to a decrease in enzyme activity and slower metabolism, while higher temperatures can actually denature proteins such as enzymes and carrier proteins, leading to cell death. As a result, microbes have a growth curve in relation to temperature with an optimal temperature at which growth rate peaks, as well as minimum and maximum temperatures where growth continues but is not as robust. For a bacterium the growth range is typically around 30 degrees.
The psychrophiles are the cold lovers, with an optimum of 15oC or lower and a growth range of -20oC to 20oC. Most of these microbes are found in the oceans, where the temperature is often 5oC or colder. They can also be found in the Arctic and the Antarctic, living in ice wherever they can find pockets of liquid water. Adaptation to the cold required evolution of specific proteins, particularly enzymes, that can still function in low temperatures. In addition, it also required modification to the plasma membrane to keep it semifluid. Psychrophiles have an increased amount of unsaturated and shorter-chain fatty acids. Lastly, psychrophiles produce cryoprotectants, special proteins or sugars that prevent the development of ice crystals that might damage the cell. Psychrotophs or cold tolerant microbes have a range of 0-35oC, with an optimum of 16oC or higher.
Humans are best acquainted with the mesophiles, microbes with a growth optima of 37oC and a range of 20-45oC. Almost all of the human microflora fall into this category, as well as almost all human pathogens. The mesophiles occupy the same environments that humans do, in terms of foods that we eat, surfaces that we touch, and water that we drink and swim in.
On the warmer end of the spectrum is where we find the thermophiles(“heat lovers”), the microbes that like high temperatures. Thermophiles typically have a range of 45-80oC, and a growth optimum of 60oC. There are also the hyperthermophiles, those microbes that like things extra spicy. These microbes have a growth optima of 88-106oC, a minimum of 65oC and a maximum of 120oC. Both the thermophiles and the hyperthermophiles require specialized heat-stable enzymes that are resistant to denaturation and unfolding, partly due to the presence of protective proteins known as chaperone proteins. The plasma membrane of these organisms contains more saturated fatty acids, with increased melting points.
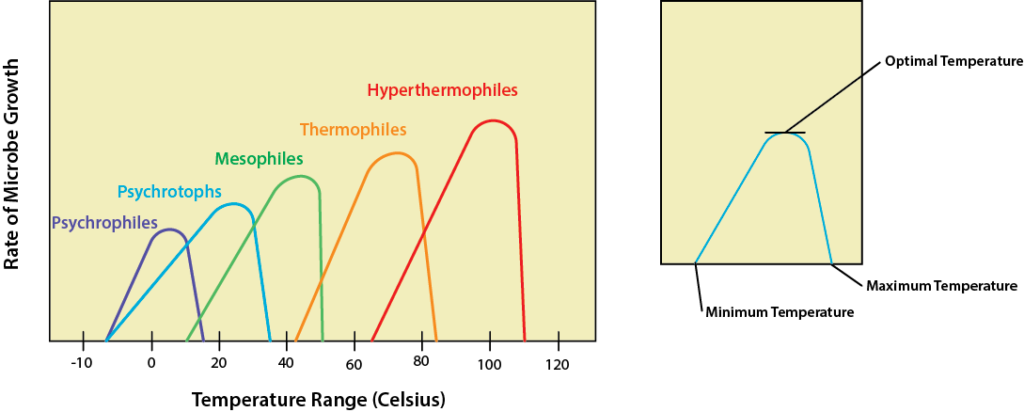
Growth Curves.
Oxygen Concentration
The oxygen requirement of an organism relates to the type of metabolism that it is using. Energy generation is tied to the movement of electrons through the electron transport chain (ETC), where the final electron acceptor can be oxygen or a non-oxygen molecule.
Organisms that use oxygen as the final electron acceptor are engaging in aerobic respiration for their metabolism. If they require the presence of atmospheric oxygen (20%) for their metabolism then they are referred to as obligate aerobes. Microaerophiles require oxygen, but at a lower level than normal atmospheric levels – they only grow at levels of 2-10%.
Organisms that can grow in the absence of oxygen are referred to as anaerobes, with several different categories existing. The facultative anaerobes are the most versatile, being able to grow in the presence or absence of oxygen by switching their metabolism to match their environment. They would prefer to grow in the presence of oxygen, however, since aerobic respiration generates the largest amount of energy and allows for faster growth. Aerotolerant anaerobes can also grow in the presence or absence of oxygen, exhibiting no preference. Obligate anaerobes can only grow in the absence of oxygen and find an oxygenated environment to be toxic.
While the use of oxygen is dictated by the organism’s metabolism, the ability to live in an oxygenated environment (regardless of whether it is used by the organism or not) is dictated by the presence/absence of several enzymes. Oxygen by-products (called reactive oxygen species or ROS) can be toxic to cells, even to the cells using aerobic respiration. Enzymes that can offer some protection from ROS include superoxide dismutase (SOD), which breaks down superoxide radicals, and catalase, which breaks down hydrogen peroxide. Obligate anaerobes lack both enzymes, leaving them little or no protection against ROS.

Oxygen and Bacterial Growth.
Pressure
The vast majority of microbes, living on land or water surface, are exposed to a pressure of approximately 1 atmosphere. But there are microbes that live on the bottom of the ocean, where the hydrostatic pressure can reach 600-1,000 atm. These microbes are the barophiles(“pressure lovers”), microbes that have adapted to prefer and even require the high pressures. These microbes have increased unsaturated fatty acids in their plasma membrane, as well as shortened fatty acid tails.
Radiation
All cells are susceptible to the damage cause by radiation, which adversely affects DNA with its short wavelength and high energy. Ionizing radiation, such as x-rays and gamma rays, causes mutations and destruction of the cell’s DNA. While bacterial endospores are extremely resistant to the harmful effects of ionizing radiation, vegetative cells were thought to be quite susceptible to its impact. That is, until the discovery of Deinococcus radiodurans, a bacterium capable of completely reassembling its DNA after exposure to massive doses of radiation.
Ultraviolet (UV) radiation also causes damage to DNA, by attaching thymine bases that are next to one another on the DNA strand. These thymine dimers inhibit DNA replication and transcription. Microbes typically have DNA repair mechanisms that allow them to repair limited damage, such as the enzyme photolyase that splits apart thymine dimers.
Key Words
osmotic pressure, passive diffusion, solute, hypotonic, hypertonic, mechanosensitive (MS) channel, compatible solute, halophile, pH, neutrophile, acidophile, alkaliphile, optimum temperature, minimum temperature, maximum temperature, psychrophile, psychrotroph, mesophile, thermophile, hyperthermophile, chaperone protein, electron transport chain (ETC), aerobic respiration, obligate aerobe, microaerophile, anaerobe, facultative anaerobe, aerotolerant anaerobe, obligate anaerobe, reactive oxygen species (ROS), superoxide dismutase (SOD), catalase, barophile, ionizing radiation, Deinococcus radiodurans, ultraviolet (UV) radiation, thymine dimmers, photolyase.
Essential Questions/Objectives
- What are all the descriptive terms used for microbes that live in different environments or the terms used for the environments that they live in? What does each term mean? In what types of environments are each microbial group found?
- What effect does solute concentration have on microbes? How can cells adapt when going from a low solute to a high solute environment and vice versa? What is a compatible solute? What microbial groups have a requirement for high solute concentrations? How do microbes differ in their response to water activity?
- How do microbes differ in their response to pH? What does pH affect in a cell and what do cells that live at high or low pH have to do to survive these conditions?
- How do microbes differ in their response to temperatures? What terms are used for these responses? If a cell is to grow at low or high temperatures, what adaptations does it need to make?
- How do microbes differ in their response to oxygen levels? Why would they differ? What enzymes are needed to adapt to environments containing differing amounts of oxygen?
- How do microbes respond to high pressure? To ionizing radiation? To UV light? What populations are resistant to these conditions?
Exploratory Questions (OPTIONAL)
- What is the largest bacterium or archaean ever discovered? What is the smallest eukaryote ever discovered?


