11.6: Vesicular Transport
- Page ID
- 16161
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)In addition to protein processing, the ER and Golgi also take care of some types of protein transport. Vesicles (membrane-bound bubbles, essentially) pinch off from the ER, Golgi, and other membranous organelles, carrying with them whatever soluble molecules were inside the fluid that was enclosed as well as any molecules embedded in that section of membrane. These vesicles then catch a ride on a molecular motor such as kinesin or myosin, and travel along the cytoskeleton until they dock at the appropriate destination and fuse with the target membrane or organelle. In general, vesicles move from the ER to the cis Golgi, from the cis to the medial Golgi, from the medial to the trans Golgi, and from the trans Golgi to the plasma membrane or other compartments. Although most movement is in this direction, there are also vesicles that move back from the Golgi to the ER, carrying proteins that were supposed to stay in the ER (e.g. PDI) and were accidentally scooped up within a vesicle.
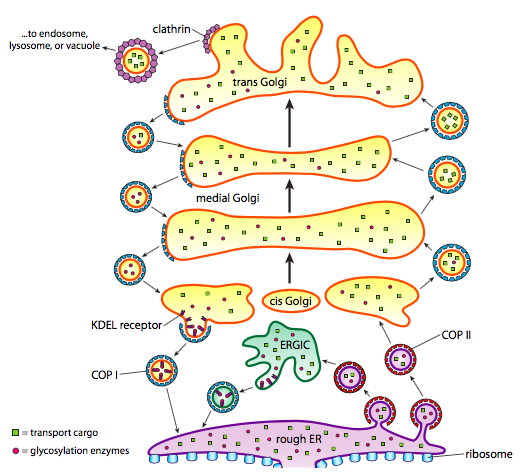
The formation of vesicles is dependent on coat proteins that will, under proper conditions, self-assemble into spherical cages. When associated with transmembrane proteins, they can pull the attached membrane along into a spherical shape also. The major types of coat proteins used in vesicle formation are COPII, COPI, and clathrin.
COPII coat proteins form the vesicles that move from ER to Golgi. COPI coat proteins are used between parts of the Golgi apparatus as well as to form vesicles going from the Golgi back to the ER. Finally, clathrin is used to form vesicles leaving the Golgi for the plasma membrane as well as for vesicles formed from the plasma membrane for endocytosis.
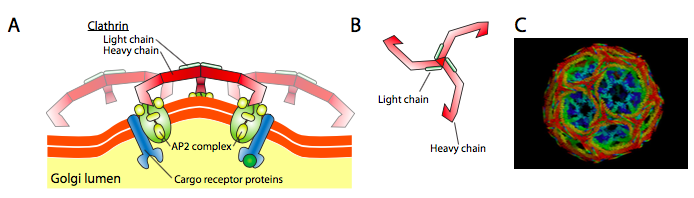
Clathrin (Figure \(\PageIndex{17}\)) is the best described of the three, and the vesicular coats are made from arrangements of clathrin triskelions (from Greek, meaning three-legged). Each triskelion is composed of three heavy chains joined together at the C-terminus, and three light chains, one associated with each heavy chain. The heavy chains of different triskelions interact along the length of their heavy chain “legs” to create a very sturdy construct. The light chains are unnecessary for vesicle formation, and are thought to help prevent accidental interactions of clathrin molecules in the cytoplasm.
There is significant similarity between the vesicle formation mechanisms using these different coat proteins, beginning with the recruitment of ARF1 (ARF stands for ADP ribosylation factor, which has nothing to do with its function here) to the membrane. This requires the ARNO-facilitated exchange of a GTP for GDP (ARNO is ARF nucleotide binding site opener). Once ARF1 has bound GTP, the conformational change reveals an N-terminal myristoyl group which inserts into the membrane. Both COPI and clathrin-coated vesicles use ARF1 and ARNO, but COPII uses similar proteins called Sar1p and Sec12p.
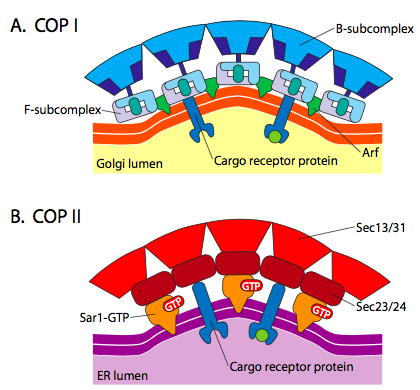
Figure \(\PageIndex{18}\). COP-coated vesicles
The ARF1 (or Sar1p) is used to recruit adapter proteins that bind to the “tail” end of membrane-bound receptor proteins. The business end of these receptors binds to car- go molecules that need to be packaged into the vesicle. The adapter proteins act as the link between the membrane (through the receptors) and the coat proteins. For clathrin, the adapter proteins are AP1 for trans-Golgi-derived vesicles and AP2 for endocytic vesicles. For COPI vesicles, the approximate homologues are the β-, γ-, δ-, and ζ- COPs while the COPII system uses Sec23p and Sec24p.
Finally, the adapters link to the actual coat proteins: clathrin, α- or ε- COP, Sec13p and Sec31p. What these proteins all have in common is that spontaneously (i.e. without any requirement for energy expenditure), they self-assemble into cage-like spherical structures. Under the electron microscope, the clathrin-coated vesicles are more sharply defined and the hexagonal and pentagonal shapes bounded by the clathrin subunits give the vesicle a “soccer ball” look. COP coatamer-coated vesicles are much fuzzier in appearance under EM.
All three types of vesicle coat proteins have the ability to spontaneously associate into a spherical construct, but only the COPI and COPII coated vesicle also spontaneously “pinch off” the membrane to release the vesicle from its originating membrane. Clathrin-coated vesicles require an external mechanism to release the vesicle (Figure \(\PageIndex{19}\)).
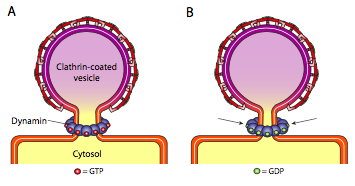
Once the vesicle has almost completed, there is still a small stalk or neck of membrane that connects the vesicle to the membrane. Around this stalk, dynamic GTP molecules aggregate in a ring/spiral construction. Dynamin molecules are globular GTPases that contract upon hydrolysis of GTP. When they associate around the vesicle stalk, each dynamin protein contracts, with the combined effect of constricting the stalk enough that the membrane pinches together, sealing off and releasing the vesicle from the originating membrane.
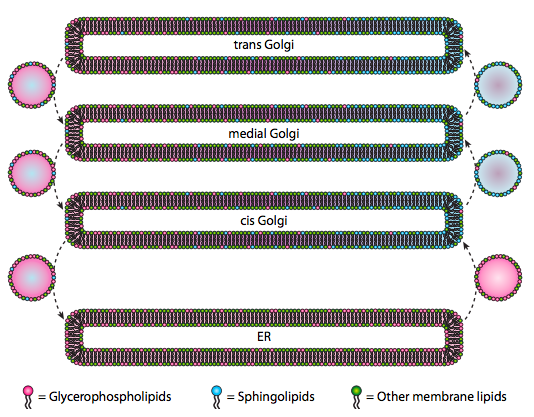
Although lipids and membranes were discussed in chapter 4, we neglected to discuss the location of their syntheses in eukaryotes. As Figure \(\PageIndex{20}\) indicates, the synthesis of certain types of lipids is segregated and exclusive. Glycerophospholipids are primarily formed in the endoplasmic reticulum, although they are also made in mitochondria and peroxisomes. In contrast, sphingolipids are not made in the ER (though their ceramide precursors are) in mammals, the necessary enzymes are found in the lumen of the cis and medial Golgi. There is evidence of anterograde and retrograde vesicular traffic between the various Golgi and ER compartments, which would theoretically indicate a redistribution of lipid types. However, the sphingolipids tend to aggregate into lipid rafts and seem to be more concentrated in anterograde-moving vesicles.
The coat proteins come off shortly after vesicular release. For clathrin, the process involves Hsc70, an ATPase. However, for COPI or COPII coated vesicles, hydrolysis of the GTP on ARF/Sar1p appears to weaken the coat protein affinity for the adapters and initiates uncoating. The GTPase activator is ARF GAP (or Sec23p) and is an integral part of the COP I (or II) coat.
The vesicles carry two categories of cargo: soluble proteins and transmembrane proteins. Of the soluble proteins, some are taken up in the vesicle by virtue of being bound to a receptor. Other proteins just happen to be in the vicinity and are scooped up as the vesicle forms. Occasionally, a protein is taken up that was not supposed to be; for example, PDI may be enclosed in a vesicle forming from the ER. It has little function in the Golgi, and is needed in the ER, so what happens to it? Fortunately, PDI and many other ER proteins have a C-terminal signal sequence, KDEL (Lysine-Aspartic Acid-Glutamic Acid-Leucine), that screams “I belong in the ER.” This sequence is recognized by KDEL receptors inside the Golgi, and binding of the KDEL proteins to the receptors triggers vesicle formation to send them back to the ER.
Secretory vesicles have a special problem with soluble cargo. If the vesicle was to rely simply on enclosing proteins within it during the formation process, it would be difficult to get high concentrations of those proteins. Many secreted proteins are needed by the organism quickly and in significant amounts, so there is a mechanism in the trans Golgi for aggregating secretory proteins. The mechanism uses aggregating proteins such as secretogranin II and chromogranin B that bring together the target proteins in large concentrated granules. These granins work best in the trans Golgi milieu of low pH and high Ca2+, so when the vesicle releases its contents outside of the cell, the higher pH and lower Ca2+ breaks apart the aggregates to release the individual proteins.
There is a consistent pH change during the maturation of the Golgi, so that as we go from ER to Golgi, each compartment has a progressively lower (more acidic) lumenal pH.
Finally, there is the question of targeting the vesicles. The vesicles are much less useful if they are tossed on a molecular freight train and dropped off at random. Therefore, there is a docking mechanism that requires a matching of the v-SNARE protein on the vesicle’s cytoplasmic surface and the t-SNARE on the cytoplasmic surface of the target membrane. Fusion of the vesicle to the membrane only proceeds if there is a match. Otherwise, the vesicle cannot fuse, and will attach to another molecular motor to head to another, hopefully correct, destination. This process is aided by tethering proteins which initially make contact with an incoming vesicle and draw it close enough to the target to test for SNARE protein interaction. Other proteins on the vesicle and target membranes then interact and if the SNAREs match, can help to “winch” the vesicle into the target membrane, whereupon the membranes fuse. An important rule of thumb to understanding vesicular fusion and also the directionality of membrane proteins and lipids, is that the cytoplasmic-facing side of a membrane is always going to be facing the cytoplasm. Therefore a protein that is eventually found on the outer surface of the cell membrane will have been inserted into the lumenal surface of the ER membrane to begin with.
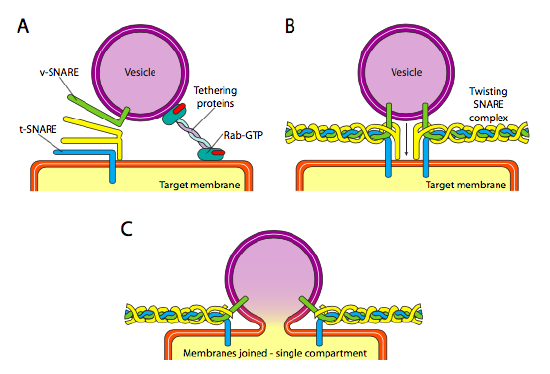
More specifically, as a vesicle approaches the target membrane, the tethering protein Rab-GTP, which is linked to the target membrane via a double geranylgeranyl lipid tail, loosely associates with the vesicle and holds it in the vicinity of the target membrane to give the SNARES a chance to work. The v-SNAREs and t-SNAREs now have the opportunity to interact and test for a match. Recently, the SNAREs have been renamed R-SNAREs and Q-SNAREs, respectively, based on conserved arginine and glutamine residues. In addition to these two primary SNAREs, at least one other SNARE is involved, together forming a bundle of four α-helices (four, not three, because at least in the best studied example, one of the SNAREs is bent around so that two of its alpha-helical domains participate in the interaction. The four helices wrap around each other and it is thought that as they do so, they pull the vesicle and the target membrane together.
The tetanus toxin, tetanospasmin, which is released by Clostridium tetani bacteria, causes spasms by acting on nerve cells, and preventing neurotransmitter release. The mechanism for this is that it cleaves synaptobrevin, a SNARE protein, so that the synaptic vesicles cannot fuse with the cell membrane. Botulinum toxin, from Clostridium botulinum, also acts on SNAREs to prevent vesicle fusion and neurotransmitter release, although it targets different neurons and so has the opposite effect: tetanus is caused by preventing the release of inhibitory neurotransmitters, while botulism is caused by preventing release of excitatory neurotransmitters.


