5.1: Basics of Energy
- Page ID
- 7829
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Source: BiochemFFA_5_1.pdf. The entire textbook is available for free from the authors at http://biochem.science.oregonstate.edu/content/biochemistry-free-and-easy

Living organisms are made up of cells, and cells contain a horde of biochemical components. Living cells, though, are not random collections of these molecules. They are extraordinarily organized or "ordered". By contrast, in the nonliving world, there is a universal tendency to increasing disorder. Maintaining and creating order in cells takes the input of energy. Without energy, life is not possible.
Oxidative Energy
The primary mechanism used by non-photosynthetic organisms to obtain energy is oxidation and carbon is the most commonly oxidized energy source. The energy released during the oxidative steps is “captured” in ATP and can be used later for energy coupling. The more reduced a carbon atom is, the more energy can be realized from its oxidation. Fatty acids are highly reduced, whereas carbohydrates are moderately so. Complete oxidation of both leads to carbon dioxide, which has the lowest energy state. Conversely, the more oxidized a carbon atom is, the more energy it takes to reduce it.
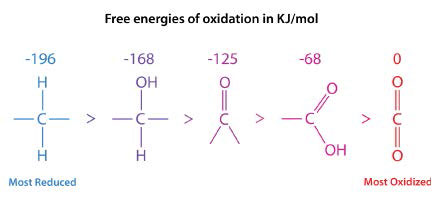
In the series shown in Figure \(\PageIndex{1}\), the most reduced form of carbon is on the left. The energy of oxidation of each form is shown above it. The reduction states of fatty acids and carbohydrates can be seen by their formulas.
- Palmitic acid: \(\ce{C16H34O2}\)
- Glucose: \(\ce{C6H12O6}\)
Palmitic acid only contains two oxygens per sixteen carbons, whereas glucose has six oxygen atoms per six carbons. Consequently, when palmitic acid is fully oxidized, it generates more ATP per carbon (128/16) than glucose (38/6). It is because of this that we use fat (contains fatty acids) as our primary energy storage material.
Figure \(\PageIndex{2}\): Photosynthesis: The primary source of biological energy. Image by Aleia Kim
Oxidation vs. Reduction in Metabolism
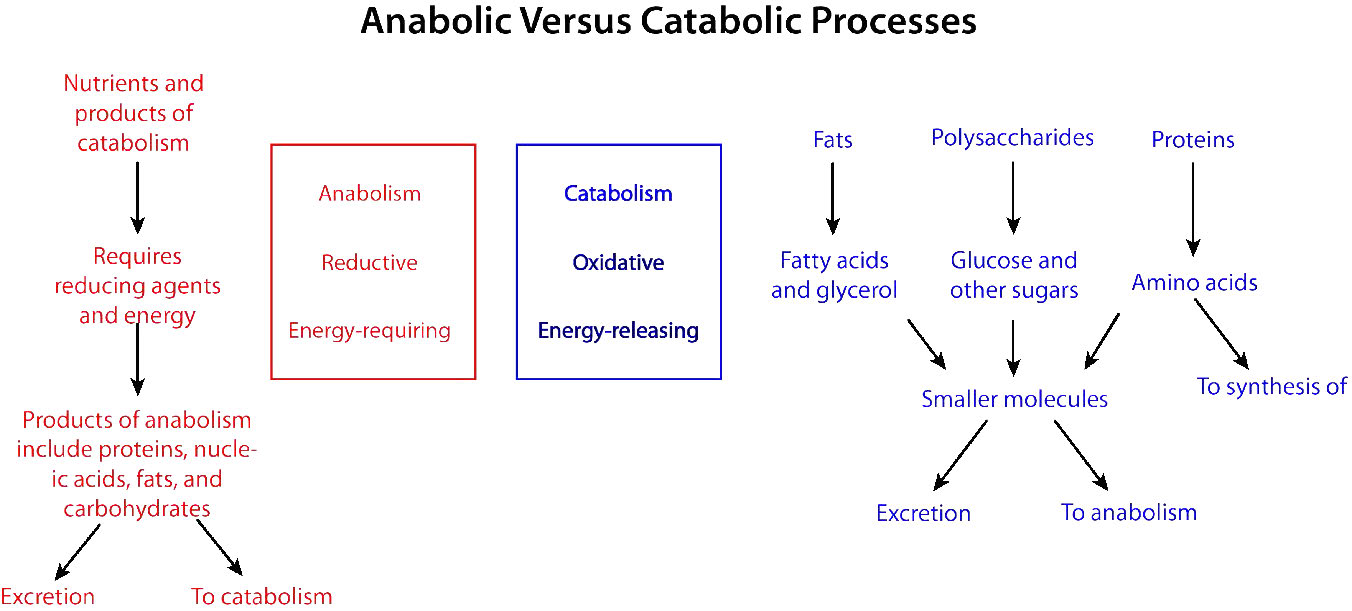
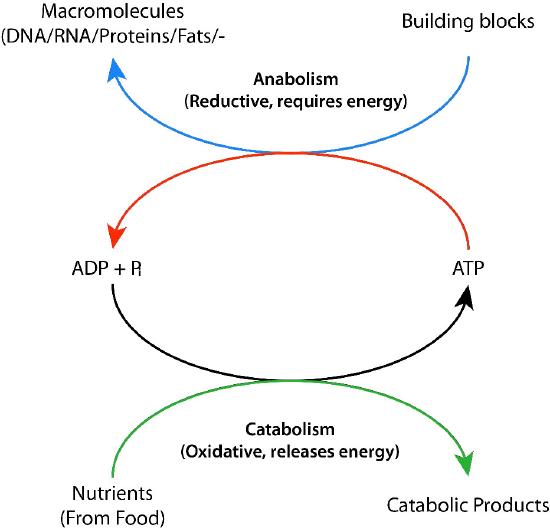
Biochemical processes that break things down from larger to smaller are called catabolic processes. Catabolic processes are often oxidative in nature and energy releasing. Some, but not all, of that energy is captured as ATP. If not all of the energy is captured as ATP, what happens to the rest of it? The answer is simple. It is released as heat and it is for this reason we get hot when we exercise.
By contrast, synthesizing large molecules from smaller ones (for example, making proteins from amino acids) is referred to as anabolism. Anabolic processes are often reductive in nature (Figures 5.3 & 5.4) and require energy input. By themselves, they would not occur, as they are reversing oxidation and decreasing entropy (making many small things into a larger one). To overcome this energy barrier, cells must expend energy. For example, if one wishes to reduce \(\ce{CO2}\) to carbohydrate, energy must be used to do so. Plants do this during the dark reactions of photosynthesis (Figure \(\PageIndex{3}\)). The energy source for the reduction is ultimately the sun. The electrons for the reduction come from water, and the \(\ce{CO2}\) is removed from the atmosphere and gets incorporated into a sugar.
Energy Coupling
The synthesis of the many molecules needed by cells needs the input of energy to occur. Cells overcome this energy obstacle by using ATP to “drive” the reaction (Figure \(\PageIndex{6}\)). The energy needed to drive reactions is harvested in very controlled conditions in enzymes. This involves a process called ‘coupling’. Coupled reactions rely on linking an energetically favorable reaction (i.e., one with a negative ∆G°’) with the reaction requiring an energy input, which has a positive ∆G°’. As long as the overall ∆G°’ of the two reactions together is negative, the reaction can proceed. Hydrolysis of ATP is a very energetically favorable reaction that is commonly linked to many energy requiring reactions in cells. Without the hydrolysis of ATP (or GTP, in some cases), the reaction would not be feasible.
Entropy and energy
Most students who have had some chemistry know about the Second Law of Thermodynamics with respect to increasing disorder of a system. Cells are very organized or ordered structures, leading some to mistakenly conclude that life somehow violates the second law. In fact, that notion is incorrect. The second law doesn’t say that entropy always increases, just that, left alone, it tends to do so, in an isolated system. Cells are not isolated systems, though, in that they obtain energy, either from the sun, if they are autotrophic, or food, if they are heterotrophic.
To counter the universal tendency towards disorder on a local scale requires energy. As an example, take a fresh deck of cards which is neatly aligned with Ace-King-Queen . . . . 4,3,2 for each suit. Throw the deck into the air, letting the cards scatter. When you pick them up, they will be more disordered than when they started. However, if you spend a few minutes (and expend a bit of energy), you can reorganize the same deck back to its previous, organized state. If entropy always increased everywhere, you could not do this. However, with the input of energy, you overcame the disorder. This illustrates an important concept: the cost of fighting disorder is energy.
Biological energy
There are, of course, other reasons that organisms need energy. Muscular contraction, synthesis of molecules, neurotransmission, signaling, thermoregulation, and subcellular movements are examples. Where does this energy come from? The currencies of energy are generally high-energy phosphate-containing molecules. ATP is the best known and most abundant, but GTP is also an important energy source (energy source for protein synthesis). CTP is involved in synthesis of glycerophospholipids and UTP is used for synthesis of glycogen and other sugar compounds. In each of these cases, the energy is in the form of potential chemical energy stored in the multi-phosphate bonds. Hydrolyzing those bonds releases the energy in them.
Of the triphosphates, ATP is the primary energy source, acting to facilitate the synthesis of the others by action of the enzyme NDPK. ATP is made by three distinct types of phosphorylation – oxidative phosphorylation (in mitochondria), photophosphorylation (in chloroplasts of plants), and substrate level phosphorylation (in enzymatically catalyzed reactions).
Gibbs free energy in Biology
ATP is generally considered the “storage battery” of cells (See also ‘Molecular Battery Backups for Muscles HERE). In order to understand how energy is captured, we must first understand Gibbs free energy and in doing so, we begin to see the role of energy in determining the directions chemical reactions take.
Gibbs free energy may be thought of as the energy available to do work in a thermodynamic system at constant temperature and pressure. Mathematically, the Gibbs free energy is given as:
\[G = H – TS\]
where \(H\) is the enthalpy, \(T\) is the temperature in Kelvin, and \(S\) is the entropy. At standard temperature and pressure, every system seeks to achieve a minimum of free energy. Thus, increasing entropy, \(S\), will reduce Gibbs free energy. Similarly, if excess heat is available (reducing the enthalpy, \(H\)), the free energy can also be reduced.
Cells must work within the laws of thermodynamics, as noted, so all of their biochemical reactions, too, are ruled by these laws. Now we shall consider energy in the cell. The change in Gibbs free energy (\(∆G\)) for a reaction is crucial, for it, and it alone, determines whether or not a reaction goes forward.
\[∆G = ∆H – T ∆S.\]
There are three cases
- ∆G < 0: the reaction proceeds as written
- ∆G = 0: the reaction is at equilibrium
- ∆G > 0: the reaction runs in reverse
For a reaction
\[\ce{aA <=> bB}\]
(where ‘a’ and ‘b’ are integers and A and B are molecules) at pH 7, ∆G can be determined by the following equation,
\[∆G = ∆G°’ + RT \ln(\frac{[B]^b}{[A]^a})\]
For multiple substrate reactions, such as
\[\ce{aA + cC <=> bB + dD}\]
\[∆G = ∆G°’ + RT \ln(\frac{[B]^b [D]^d}{[A]^a[C]^c})\]
The ∆G°’ term is called the change in Standard Gibbs Free energy, which is the change in energy that occurs when all of the products and reactants are at standard conditions and the pH is 7.0. It is a constant for a given reaction.
In simple terms, we can collect all of the terms of the numerator together and call them {Products} and all of the terms of the denominator together and call them {Reactants},
\[∆G = ∆G°’ + RT \ln(\frac{\rm{\{Products\}}}{\rm{\{Reactants\}}})\]
For most biological systems, the temperature, T, is a constant for a given reaction. Since ∆G°’ is also a constant for a given reaction, the ∆G is changed almost exclusively as the ratio of {Products}/{Reactants} changes.
Importance of ∆G°’
If one starts out at standard conditions, where everything except protons is at 1M, the RTln({Products}/{Reactants}) term is zero, so the ∆G°’ term equals the ∆G, and the ∆G°’ determines the direction the reaction will take (only under those conditions). This is why people say that a negative ∆G°’ indicates an energetically favorable reaction, whereas a positive ∆G°’ corresponds to an unfavorable one.
Increasing the ratio of {Products}/{Reactants} causes the value of the natural log (ln) term to become more positive (less negative), thus making the value of ∆G more positive. Conversely, as the ratio of {Products}/{Reactants} decreases, the value of the natural log term becomes less positive (more negative), thus making the value of ∆G more negative.
System response to stress
Intuitively, this makes sense and is consistent with Le Chatelier’s Principle – a system responds to stress by acting to alleviate the stress. If we examine the ∆G for a reaction in a closed system, we see that it will always move to a value of zero (equilibrium), no matter whether it starts with a positive or negative value.
Another type of free energy available to cells is that generated by electrical potential. For example, mitochondria and chloroplasts partly use Coulombic energy (based on charge) from a proton gradient across their membranes to provide the necessary energy for the synthesis of ATP. Similar energies drive the transmission of nerve signals (sodium and potassium gradients) and the movement of some molecules in secondary active transport processes across membranes (e.g., H+ differential driving the movement of lactose). From the Gibbs free energy change equation,
\[∆G = ∆H – T∆S\]
it should be noted that an increase in entropy will help contribute to a decrease in ∆G. This happens, for example when a large molecule is being broken into smaller pieces or when the rearrangement of a molecule increases the disorder of molecules around it. The latter situation arises in the hydrophobic effect, which helps drive the folding of proteins.
Chemical and electrical potential
It is said that absence makes the heart grow fonder. We won’t tackle that philosophical issue here, but we will say that separation provides potential energy that cells can and do harvest. The lipid bilayer of cell and (in eukaryotic cells) organelle membranes provide the necessary barrier for separation.
Impermeable to most ions and polar compounds, biological membranes are essential for processes that generate cellular energy. Consider Figure 5.8. A lipid bilayer separates two solutions with different concentrations of a solute. There is a greater concentration of negative ions in the bottom and a greater concentration of positive ions on the top.
Whenever there is a difference in concentration of molecules across a membrane, there is said to be a concentration gradient across it. A difference in concentration of ions across a membrane also creates a charge (or electrical) gradient. Because there is a difference in both the chemical concentration of the ions and in the charge on the two sides of the membrane, this is described as an electrochemical gradient (Figures 5.8 -5.10).
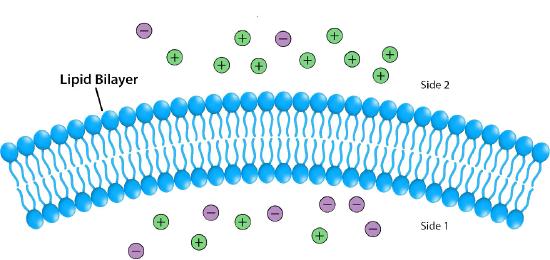
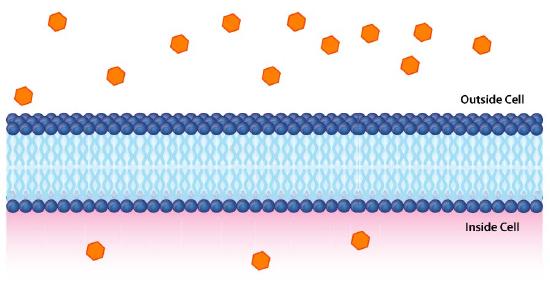
Potential energy
Such gradients function like batteries and contain potential energy. When the potential energy is harvested by cells, they can create ATP, transmit nerve signals, pump molecules across membranes, and more. It is important, therefore, to understand how to calculate the potential energy of electrochemical gradients.
First, we consider chemical (solute) gradients. In Figure 5.9, two concentrations of glucose are separated by a lipid bilayer. Let’s assume C2 be the concentration of glucose inside the cell (bottom) and C1 be the glucose concentration outside (top). The Gibbs free energy associated with moving glucose in the direction of C2 (into the cell) is given by
∆G = RTln[C2/C1]
To move it in the direction of C1 (to the outside of the cell) the expression would be
\[∆G = RT\ln[C_1/C_2]\]
Since C2 is smaller than C1 (i.e., there are fewer glucose molecules inside the cell) then the ∆G is negative and diffusion would be favored into the cell, if the glucose could traverse the bilayer.
Conversely, if C2 was greater than C1 (more glucose was in the cell than outside) the ∆G would be positive, so movement in the direction of C2 would not be favored and instead the glucose would tend to move towards C1 , that is, out of the cell.
If C2 = C1, with the same concentration of glucose inside and outside, then the ∆G would be zero and there would be no net movement, as the system would be at equilibrium.
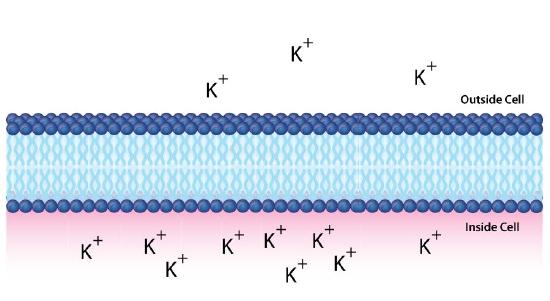
In the example above, we considered glucose, which is an uncharged molecule. When ions are involved, their charges must also be taken into consideration. Figure \(\PageIndex{1}\)0 depicts a similar situation across a lipid bilayer. In this case, a difference of concentration and charge exists. There are more positive charges inside the cell than outside.
Using C2 to indicate the concentration of materials inside the cell and C1 for the concentration outside the cell (as before), then the free energy for movement of an ion from top to bottom is given by the following equation
\[∆G = RT\ln[C_2/C_1] + ZF∆ψ\]
Note here that this equation must take into consideration both the concentration differences and the charge differences. Z refers to the charge of the transported species, F is the Faraday constant (96,485 Coulombs/mol), and ∆ψ is the electrical potential difference (voltage difference) across the membrane.
If we were to calculate the ∆G for movement of the potassium ion from top to bottom, it would be positive, since [C2/C1] is greater than 1 (making for a positive ln term), and the ZF∆ψ is positive because positively charged ions (Z) are moving against a positive charge gradient given by ∆ψ (greater concentration at the target (bottom) than the starting point (top)). If we were to calculate the concentration of ions moving from bottom to top, then the ln term would be negative (C2<C1) and the ZF∆ψ would be negative as well (Z=positive, but ∆ψ negative).
Reduction Potential
In discussing chemical potential, we must also consider reduction potential. Reduction potential measures the tendency of a chemical to be reduced by electrons. It is also designated by several other names/variables. These include redox potential, oxidation/reduction potential, ORP, pE, ε, E, and Eh.
Reduction potential is measured in volts, or millivolts. A substance with a higher reduction potential will have a greater tendency to accept electrons and be reduced. If two substances are mixed in an aqueous solution, the one with the greater (more positive) reduction potential will tend to take electrons away, thus being reduced, from the one with the lower reduction potential, which becomes oxidized.
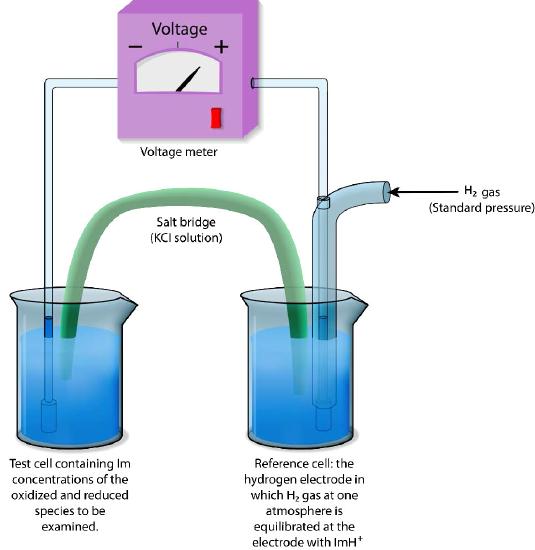
Relative measures
Absolute reduction potentials are difficult to measure, so reduction potentials are typically defined relative to a reference electrode. In aqueous solutions, reduction potentials are measured as the potential difference between an inert sensing electrode (typically platinum) in contact with the test solution and a stable reference electrode (measured as a Standard Hydrogen Electrode: SHE) as shown in Figure \(\PageIndex{1}\)1. The standard of reference for measurement is the half-reaction
H+ + e— → ½ H2
The electrode where this reaction occurs (referred to as a half-cell) is given the value of E° (Standard Reduction Potential) of 0.00 volts. The hydrogen electrode is connected via an external circuit to another half cell containing a mixture of the reduced and oxidized species of another molecule (for example, Fe++ and Fe+++) at 1M each and standard conditions of temperature (25°C) and pressure (1 atmosphere).
Direction and voltage measured
The direction and magnitude of electron movement is then measured. If the test mixture takes electrons from the hydrogen electrode, the sign of the voltage is positive and if the direction is reversed, the voltage is negative.
Thus, compounds which have greater affinity for electrons than hydrogen will register a positive voltage and negative voltages correspond to compounds with lesser affinity for electrons than hydrogen.
Movement of electrons
Under standard conditions, electrons will move from compounds generating lower voltages to ones generating higher (more positive) voltages. Just as the standard Gibbs free energy change is the Gibbs free energy change under standard conditions, so, too, is the standard reduction potential E° the reduction potential E under standard conditions.
The actual reduction potential of a half cell will vary with the concentration of each chemical species in the cell. The relationship between the reduction potential E and the standard reduction potential E° is given by the following equation (also called the Nernst equation)
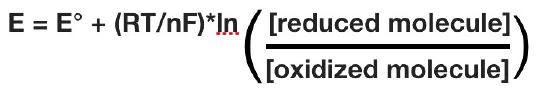
where F is the Faraday constant (96,480 J/(Volts*moles), R is the gas constant (8.315 J/(moles*K), n is the number of moles of electrons being transferred, and T is the absolute temperature in Kelvin.
At 25°C, this equation becomes
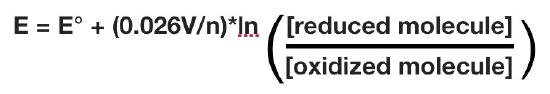
As for Gibbs free energy, it is useful to measure values at conditions found in cells. This means doing measurements at pH = 7, which differs from having all species at 1M.
Adjustment
Because of this adjustment, a slightly different standard reduction potential is defined and we designate it by E°’, just as we defined a special standard Gibbs free energy change at pH 7 as ΔG°’.
There is a relationship between the change in Gibbs free energy ΔG and the change in reduction potential (ΔE). It is
\[ΔG = -nFΔE\]
Similarly, the relation between the change in standard Gibbs free energy and the change in standard reduction potential is
\]ΔG°’ = -nFΔE°’\]
Energy Storage in Triphosphates
Movie 5.1: ATP: The fuel of the cell
Formation of triphosphates, like ATP, is essential to meeting the cell’s energy needs for synthesis, motion, and signaling. In a given day, an average human body makes and breaks down more than its weight in triphosphates. This is especially remarkable considering that there is only about 250 g of the molecule present in the body at any given time. Energy in ATP is released by hydrolysis of a phosphate from the molecule.
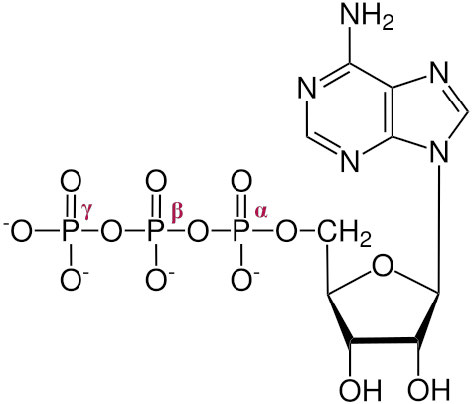
The three phosphates, starting with the one closest to the sugar are referred to as α, β, and γ (Figure \(\PageIndex{1}\)2). It is the γ phosphate that is cleaved in hydrolysis and the product is ADP. In a few reactions, the bond between the α and β is cleaved. When this happens, a pyrophosphate (β linked to γ) is released and AMP is produced. This latter reaction to produce AMP releases more energy (ΔG°’ = -45.6 kJ/mol) than the first reaction which produces ADP (ΔG°’ = -30.5 kJ/mol).
Since triphosphates are the “currency” that meet immediate needs of the cell, it is important to understand how triphosphates are made. There are three phosphorylation mechanisms – 1) substrate level; 2) oxidative; and 3) photophosphorylation. We consider them here individually.
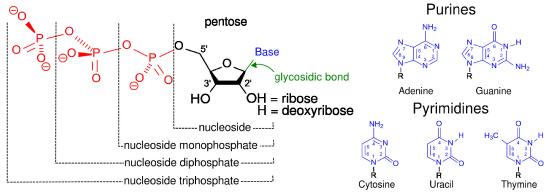
Substrate level phosphorylation
The easiest type of phosphorylation to understand is that which occurs at the substrate level. This type of phosphorylation involves the direct synthesis of ATP from ADP and a high energy intermediate, typically a phosphate-containing molecule. Substrate level phosphorylation is a relatively minor contributor to the total synthesis of triphosphates by cells. An example substrate phosphorylation comes from glycolysis.
Phosphoenolpyruvate (PEP) + ADP ⇌ Pyruvate + ATP
This reaction has a very negative ∆G°’ (-31.4 kJ/mol), indicating that the PEP contains more energy than ATP, thus tending to energetically favor ATP’s synthesis. Other triphosphates can be made by substrate level phosphorylation, as well. For example, GTP can be synthesized by the following citric acid cycle reaction.
Succinyl-CoA + GDP + Pi ⇌ Succinate + GTP + CoA-SH
Triphosphates can be interchanged readily in substrate level phosphorylations catalyzed by the enzyme Nucleoside Diphosphate Kinase (NDPK). A generalized form of the reactions catalyzed by this enzyme is as follows:
XTP + YDP ⇌ XDP + YTP
where X = adenosine, cytidine, uridine, thymidine, or guanosine and Y can be any of these as well. Further, XTP and YDP can be any of the deoxynucleotides as well.
Last, an unusual way of synthesizing ATP by substrate level phosphorylation is via the reaction catalyzed by adenylate kinase
2 ADP ⇌ ATP + AMP
ATP source
This reaction is an important means of generating ATP when the cell doesn’t have other sources of energy. Accumulation of AMP resulting from this reaction activates enzymes, such as phosphofructokinase, of glycolysis, which will catalyze reactions to give the cell additional, needed energy.
It is important to note that enzymes cannot make reactions happen that are energetically unfavorable. Enzymes speed reactions, but do not change their direction. Cells are thus bound by the rules of Gibbs free energy. So, how do energetically unfavorable reactions happen in a cell?
Reaction coupling
Reactions that are energetically unfavorable, can be made favorable by coupling them with the hydrolysis of ATP, a very energetically favorable reaction. There are numerous parallels in the “real world.” Movement of automobiles is energetically unfavorable, but coupling movement of the automobile to oxidation of gasoline makes an unfavorable process favorable. Another approach to making an unfavorable reaction favorable is to manipulate the concentration of reactants and products. Consider the reaction below, which occurs in pyrimidine nucleotide metabolism:
orotate + PRPP ⇌ OMP + PPi
The ΔG°’ for this reaction is -0.8 kJ/mol, meaning that if one starts with equal concentrations of reactants and products, at equilibrium, there will be a small excess of products. In the cell, however, this reaction moves strongly to the right (ΔG = very negative). Given that the ΔG°’ is very close to zero, a very negative ΔG can only occur if the concentrations of reactants and products are altered, since
\[ΔG = ΔG°’ + RT \ln(\frac{[\rm{OMP}][\rm{PP_i}]}{[\rm{Orotate}][\rm{PRPP}]})\]
Manipulation is exactly what happens here. The key item whose concentration is adjusted in this reaction is the pyrophosphate (PPi). This is possible because cells contain an enzyme called pyrophosphorylase that catalyzes the following reaction
PPi + H2O ⇌ 2 Pi
Hydrolysis of pyrophosphate is very energetically favored, causing the PPi produced in the reaction to be quickly hydrolyzed. As a result, the concentration of PPi in the cell is kept very low. A low concentration of a product (PPi) causes the natural log (ln) term of the orotate equation to become more negative, driving the ΔG term for the overall reaction to become much more negative.
Pushing and pulling
Reactions that yield pyrophosphate as a product are produced in the synthesis of DNA and RNA, as well as many other molecules. As shown in the previous example, this pyrophosphate is rapidly hydrolyzed, causing the overall reaction to move in the direction of pyrophosphate production. When reactants are removed/reduced in a metabolic reaction to decrease the concentration of a product, we say that the reaction is “pulled”, to represent the increase in the forward reaction as a result of product depletion.
Pushing happens when reactants in a reaction are added/increased. This too has the effect of reducing the ΔG of a reaction and making it more favorable because the ratio of [Products]/[Reactants] is decreased with increasing [Reactants]. Pushing and pulling of reactions are additional tools for cells to overcome energy barriers, just like coupling energetically favorable processes to energetically unfavorable ones.


