Photophosphorylation: Anoxygenic and Oxygenic*#
- Page ID
- 8202
( \newcommand{\kernel}{\mathrm{null}\,}\)
Photophosphorylation an Overview:
Photophosphorylation is the process of transferring the energy from light into chemicals, in particular ATP. The evolutionary roots of photophosphorylation are likely in the anaerobic world, between 3 billion and 1.5 billion years ago, when life was abundant in the absence of molecular oxygen. Photophosphorylation probably evolved relatively shortly after electron transport chains and anaerobic respiration began to provide metabolic diversity. The first step of the process involves the absorption of a photon by a pigment molecule. Light energy is transferred to the pigment and promotes electrons into a higher quantum energy state - something biologists term an "excited state". Note the use of anthropomorphism here, the electrons are not "excited" in the classic sense and aren't all of a sudden hopping all over or celebrating their promotion. They are simply in a higher energy quantum state. In this state, the electrons are colloquially said to be "energized". While in the "excited" state, the pigment now has a much lower reduction potential and can donate the "excited" electrons to other carriers with greater reduction potentials. These electron acceptors may in turn become donors to other molecules with greater reduction potentials and in so doing form an electron transport chain.
As electrons pass from one electron carrier to another via red/ox reactions, these exergonic transfers can be coupled to the endergonic transport (or pumping) of protons across a membrane to create an electrochemical gradient. This electrochemical gradient generates a proton motive force whose exergonic drive to reach equilibrium can be coupled to the endergonic production of ATP, via ATP synthase. As we will seen in more detail, the electrons involved in this electron transport chain can have one of two fates: (1) they may be returned to their initial source in a process called cyclic photophosphorylation or (2) they can be deposited onto a close relative of NAD+ called NADP+. If the electrons are deposited back on the original pigment in a cyclic process, the whole process can start over. If, however, the electron is deposited onto NADP+ to form NADPH (**shortcut note - we didn't explicitly mention any protons but assume it is understood that they are also involved**) the original pigment must regain an electron from somewhere else. This electron must come from a source with a smaller reduction potential than the oxidized pigment and depending on the system there are different possible sources, including H2O, reduced sulfur compounds such as SH2 and even elemental S0.
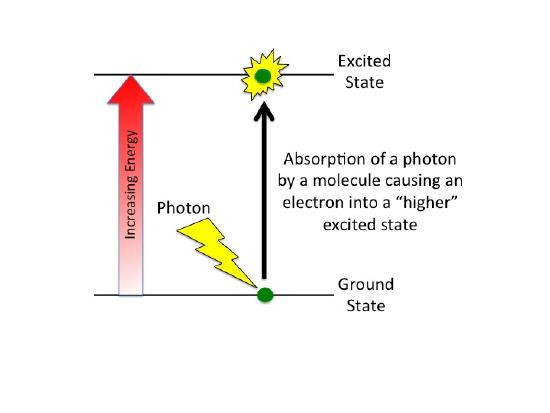
What happens when a compound absorbs a photon of light?
When a compound absorbs a photon of light, the compound is said to leave its ground state and become "excited".
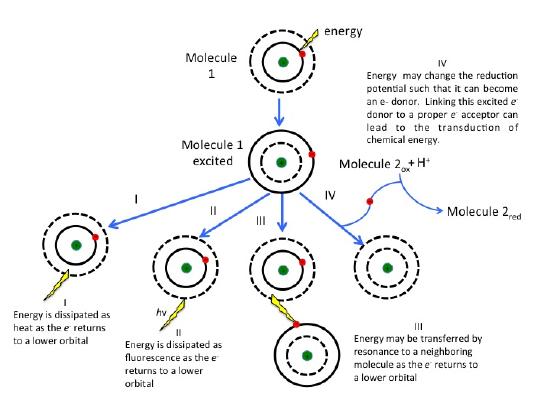
What are the fates of the "excited" electron? There are four possible outcomes, which are schematically diagrammed in the figure below. These options are:
- The electron can relax to a lower quantum state, transferring energy as heat.
- The electron can relax to a lower quantum state and transfer energy into a photon of light - a process known as fluorescence .
- The energy can be transferred by resonance to a neighboring molecule as the e- returns to a lower quantum state.
- The energy can change the reduction potential such that the molecule can become an e- donor. Linking this excited e- donor to a proper e- acceptor can lead to an exergonic electron transfer. In other words, the excited state can be involved in Red/Ox reactions.
As the excited electron decays back to its lower energy state, the energy can be transferred in a variety of ways. While many so called antenna or auxiliary pigments absorb light energy and transfer it to something known as a reaction center (by mechanisms depicted in option III in the figure above it is what happens at the reaction center that we are most concerned with (option IV in the figure above). Here a chlorophyll or bacteriochlorophyll molecule absorbs a photon's energy and an electron is excited. This energy transfer is sufficient to allow the reaction center to donate the electron in a redox reaction to a second molecule. This initiates the electron transport reactions. The result is an oxidized reaction center that must now be reduced in order to start the process again. How this happens is the basis of electron flow in photophosphorylation and will be described in detail below.
Simple Photophosphorylation Systems: Anoxygenic photophosphorylation
Introduction
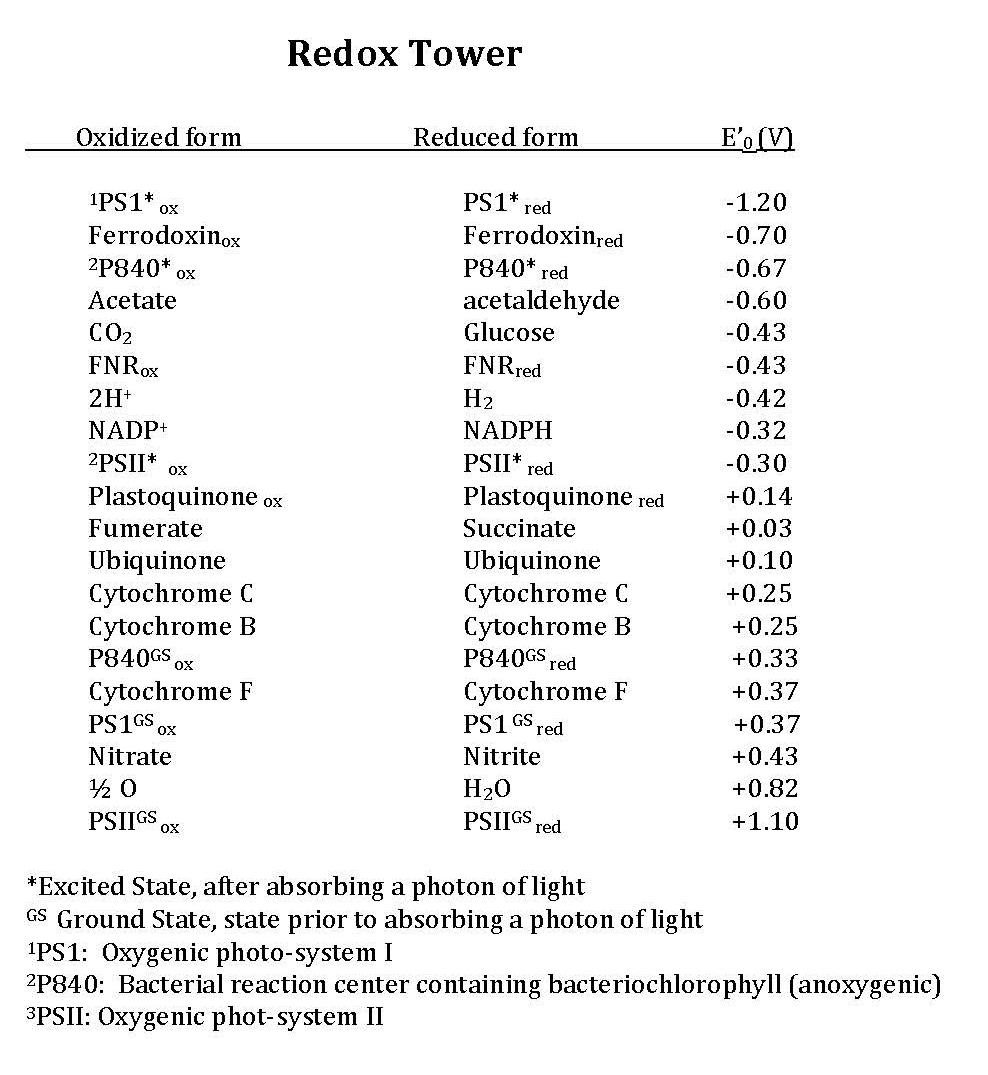
Early in the evolution of photophosphorylation, these reactions evolved in anaerobic environments where there was very little molecular oxygen available. Two sets of reactions evolved under these conditions, both directly from anaerobic respiratory chains as described previously. These are known as the light reactions because they require the activation of an electron (an "excited" electron) from the absorption of a photon of light by a reaction center pigment, such as bacteriochlorophyll. The light reactions are categorized either as cyclic or as noncyclic photophosphorylation, depending upon the final state of the electron(s) removed from the reaction center pigments. If the electron(s) return to the original pigment reaction center, such as bacteriochlorophyll, this is cyclic photophosphorylation; the electrons make a complete circuit and is diagramed in a figure titled "cyclic electron flow". If the electron(s) are used to reduce NADP+ to NADPH, the electron(s) are removed from the pathway and end up on NADPH, this process is referred to as noncyclic since the electrons are no longer part of the circuit. In this case the reaction center must be re-reduced before the process can happen again. Therefore, an external electron source is required for noncylic photophosphorylation. In these systems reduced forms of Sulfur, such as H2S, which can be used as an electron donor and is diagrammed in the figure titled "non-cyclic electron flow". To help you better understand the similarities of photophosphorylation to respiration, a redox tower has been provided that contains many commonly used compounds involved with photosphosphorylation.
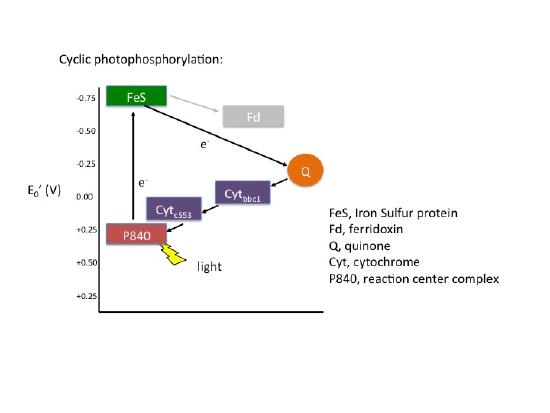
Cyclic Photophosphorylation
Cyclic Photophosphorylation
In cyclic photophosphorylation the bacteriochlorophyllred molecule absorbs enough light energy to energize and eject an electron to form bacteriochlorophyllox. The electron reduces a carrier molecule in the reaction center which in turn reduces a series of carriers via redox reactions. These carriers are the same carriers found in respiration. If the change in reduction potential from the various redox reactions are sufficiently large, protons, H+ can be translocated across a membrane. Eventually the electron is used to reduce bacteriochlorophyllox(making a complete loop) and the whole process can start again. This flow of electrons is cyclic and is therefore said to drive a processed called cyclic photophosphorylation. The electrons make a complete cycle: bacteriochlorophyll is the initial source of electrons and is the final electron acceptor. ATP is produced via the F1F0 ATPase. The schematic in the figure immediately below demonstrates how cyclic electron flow and thus cyclic photophosphorylation works.
| Cyclic Electron Flow |
|---|
Note: Possible Discussion
The figure of cyclic photophosphorylation above depicts the flow of electrons in a respiratory chain. How does this process help generate ATP?
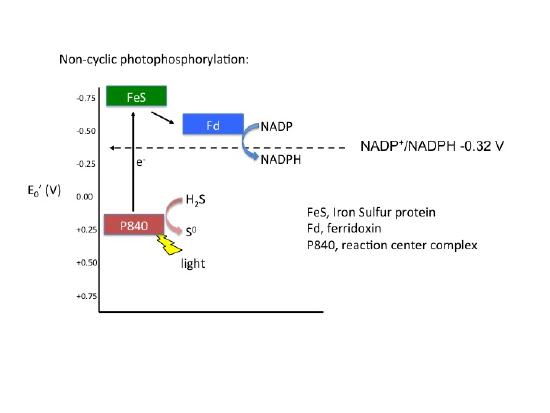
Non-cyclic photophosphorylation
Non-cyclic photophosphorylation
In cyclic photophosphorylation electrons cycle from bacteriochlorophyll (or chlorophyll) to a series of electron carriers and eventually back to bacteriochlorophyll (or chlorophyll): there is theoretically no net loss of electrons; they stay in the system. In non-cyclic photophosphorylation electrons are removed from the photosystem and redox chain and they eventually end up on NADPH. That means there needs to be a source of electrons, a source that has a smaller reduction potential than bacteriochlorophyll (or chlorophyll) that can donate electrons to bacteriochlorophyllox to reduce it. An electron tower is proved above so you can see what compounds can be used to reduce the oxidized form of bacteriochlorophyll. The second requirement, is that when bacteriochlorophyll becomes oxidized and the electron is ejected it must reduce a carrier that has a greater reduction potential than NADP/NADPH (see the electron tower). In this case, electrons can flow from energized bacteriochlorophyll to NADP forming NADPH and oxidized bacteriochlorophyll. Electrons are lost from the system and end up on NADPH, to complete the circuit bacteriochlorophyllox is reduced by an external electron donor, such as H2S or elemental S0.
| Non-cyclic Electron Flow |
|---|
Note: Possible Discussion
It should be noted that for bacterial photophosphorylation pathways, for each electron donated from a reaction center (remember only one electron is actually donated/reaction center (or chlorophyl molecule), the resulting output from that electron transport chain is either the formation of NADPH (requires 2 electrons)or ATP can be made, NOT not both. In other words, the path the electrons take in the ETC can have one or two possible outcomes. This puts limits on the versatility of the bacterial anoxygenic photosynthetic systems. But what would happen if there evolved a process that utilized both systems, that is a cyclic and non-cyclic photosynthetic pathway? That is, if both ATP and NADPH could be formed from from a single input of electrons? A second limitation is that these bacterial systems require compounds such as reduced sulfur to act as electron donors to reduce the oxidized reaction centers, not necessarily widely found compounds. What would happen if a chlorophyllox molecule would have a reduction potential higher (more positive) than that of the molecular the O2/H2O reaction? Answer, a planetary game changer.
Oxygenic Photophosphorylation
Generation of NADPH and ATP
The overall function of light-dependent reactions is to transfer solar energy into chemical compounds, largely the the molecules NADPH and ATP. This energy supports the light-independent reactions and fuels the assembly of sugar molecules. The light-dependent reactions are depicted in the next two figures. Protein complexes and pigment molecules work together to produce NADPH and ATP.
Note: Possible Discussion
Step back a little. Why is it a reasonable goal to want to make NADPH and ATP? In the discussion of glycolysis and the TCA cycle the goal was to make ATP and NADH. What is the key difference? Perhaps how those molecules will be used? Something else?
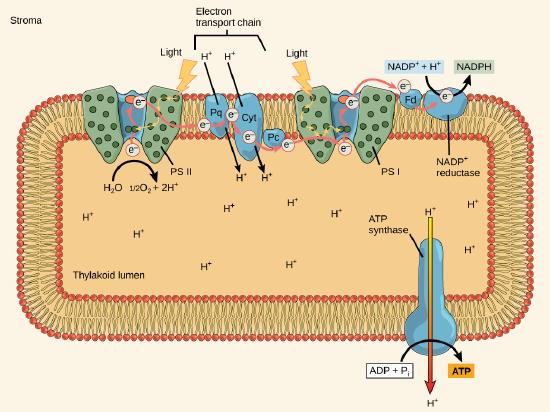
The actual step that transfers light energy into the biomolecule takes place in a multiprotein complex called a photosystem, two types of which are found embedded in the thylakoid membrane, photosystem II (PSII) and photosystem I (PSI). The two complexes differ on the basis of what they oxidize (that is, the source of the low-energy electron supply) and what they reduce (the place to which they deliver their energized electrons).
Both photosystems have the same basic structure; a number of antenna proteins to which the chlorophyll molecules are bound surround the reaction center where the photochemistry takes place. Each photosystem is serviced by the light-harvesting complex, which passes energy from sunlight to the reaction center; it consists of multiple antenna proteins that contain a mixture of 300–400 chlorophyll a and b molecules as well as other pigments like carotenoids. The absorption of a single photon - a distinct quantity or “packet” of light - by any of the chlorophylls pushes that molecule into an excited state. In short, the light energy has now been captured by biological molecules but is not yet stored in any useful form. The captured energy is transferred from chlorophyll to chlorophyll until eventually (after about a millionth of a second), it is delivered to the reaction center. Up to this point, only energy has been transferred between molecules, not electrons.
The reaction center contains a pair of chlorophyll a molecules with a special property. Those two chlorophylls can undergo oxidation upon excitation; they can actually give up an electron in a process called a photoactivation. It is at this step in the reaction center, this step in photophosphorylation, that light energy is transferred into an excited electron. All of the subsequent steps involve getting that electron onto the energy carrier NADPH for delivery to the Calvin cycle where the electron can be deposited onto carbon for long-term storage in the form of a carbohydrate.
The electron transport chain
PSII and PSI are two major components of the photosynthetic electron transport chain, which also includes the cytochrome complex. The cytochrome complex, an enzyme composed of two protein complexes, transfers the electrons from the carrier molecule plastoquinone (Pq) to the protein plastocyanin (Pc), thus enabling both the transfer of protons across the thylakoid membrane and the transfer of electrons from PSII to PSI.
The reaction center of PSII (called P680) delivers its high-energy electrons, one at a time, to a primary electron acceptor, and through the electron transport chain (Pq to cytochrome complex to plastocyanine) to PSI. P680’s missing electron is replaced by extracting an electron from water; thus, water is split and PSII is re-reduced after every photoactivation step. Splitting one H2O molecule releases two electrons, two hydrogen atoms, and one atom of oxygen. Splitting two molecules of water is required to form one molecule of diatomic O2 gas. In plants, about 10 percent of that oxygen is used by mitochondria in the leaf to support oxidative phosphorylation. The remainder escapes to the atmosphere where it is used by aerobic organisms to support respiration.
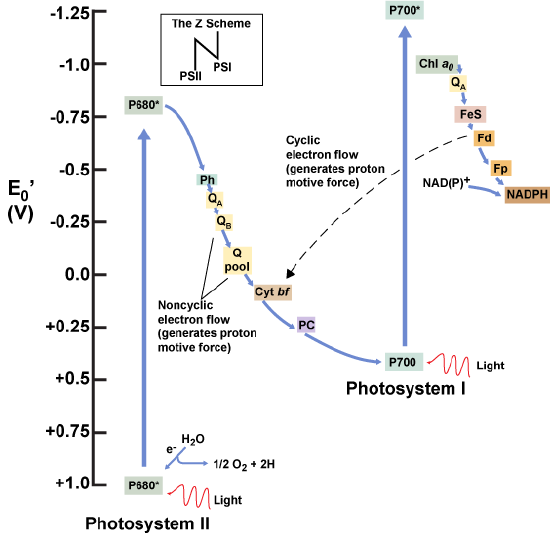
As electrons move through the proteins that reside between PSII and PSI, they take part in exergonic redox transfers. The free energy associated with the exergonic redox reaction is coupled to the endergonic transport of protons from the stromal side of the membrane to the thylakoid lumen. Those hydrogen ions, plus the ones produced by splitting water, accumulate in the thylakoid lumen and create a proton motive force that will be used to drive the synthesis of ATP in a later step. Since the electrons on PSI now have a greater reduction potential than when they started their trek (it is important to note that unexcited PSI has a greater redox potential than NADP+/NADPH), they must be re-energized in PSI before getting deposited onto NADP+. Therefore, to complete this process another photon must be absorbed by the PSI antenna. That energy is transferred to the PSI reaction center (called P700). P700 is oxidized and sends an electron through several intermediate redox steps to NADP+ to form NADPH. Thus, PSII captures the energy in light and couples its transfer via redox reactions to the creation of a proton gradient. As already noted, the exergonic and controlled relaxation of this gradient can be coupled to the synthesis of ATP. PSI captures energy in light and couples that, through a series of redox reactions, to reduce NADP+ into NADPH. The two photosystems work in concert, in part, to guarantee that the production of NADPH will be in the right proportion to the production of ATP. Other mechanisms exist to fine tune that ratio to exactly match the chloroplast’s constantly changing energy needs.
Figure 8: A diagram depicting the flow of electrons and the redox potentials of their carriers in oxygenic photosynthetic systems expressing both photosystem I and photosystem II.
Attribution: Marc T. Facciotti (own work)