6.12: Fatty Acid Synthesis
- Page ID
- 2999
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Synthesis of fatty acids occurs in the cytoplasm and endoplasmic reticulum of the cell and is chemically similar to the beta-oxidation process, but with a couple of key differences. The first of these occur in preparing substrates for the reactions that grow the fatty acid. Transport of acetyl-CoA from the mitochondria occurs when it begins to build up. Two molecules can play roles in moving it to the cytoplasm – citrate and acetylcarnitine. Joining of oxaloacetate with acetyl-CoA in the mitochondrion creates citrate which moves across the membrane, followed by action of citrate lyase in the cytoplasm of the cell to release acetyl-CoA and oxaloacetate. Additionally, when free acetyl-CoA accumulates in the mitochondrion, it may combine with carnitine and be transported out to the cytoplasm.
Starting with two acetyl-CoA, one is converted to malonyl-CoA by carboxylation catalyzed by the enzyme acetyl-CoA carboxylase (ACC), the only regulatory enzyme of fatty acid synthesis (Figure \(\PageIndex{1}\)). Next, both molecules have their CoA portions replaced by a carrier protein known as ACP (acyl-carrier protein) to form acetyl-ACP and malonyl-ACP. Joining of a fatty acyl-ACP (in this case, acetyl-ACP) with malonyl-ACP splits out the carboxyl that was added and creates the intermediate at the upper right in the figure at left.

Figure \(\PageIndex{1}\): Fatty Acid Synthesis
From this point forward, the chemical reactions resemble those of beta oxidation reversed. First, the ketone is reduced to a hydroxyl using NADPH. In contrast to the hydroxylated intermediate of beta oxidation, the beta intermediate here is in the D-configuration. Next, water is removed from carbons 2 and 3 of the hydroxyl intermediate to produce a trans doubled bonded molecule. Last, the double bond is hydrogenated to yield a saturated intermediate. The process cycles with the addition of another malonyl-ACP to the growing chain until ultimately an intermediate with 16 carbons is produced (palmitoyl-CoA). At this point, the cytoplasmic synthesis ceases.
Enzymes of Fatty Acid Synthesis
Acetyl-CoA carboxylase, which catalyzes synthesis of malonyl-CoA, is the only regulated enzyme in fatty acid synthesis. Its regulation involves both allosteric control and covalent modification. The enzyme is known to be phosphorylated by both AMP Kinase and Protein Kinase A. Dephosphorylation is stimulated by phosphatases activated by insulin binding. Dephosphorylation activates the enzyme and favors its assembly into a long polymer, while phosphorylation reverses the process.Citrate acts as an allosteric activator and may also favor polymerization. Palmitoyl-CoA allosterically inactivates it.
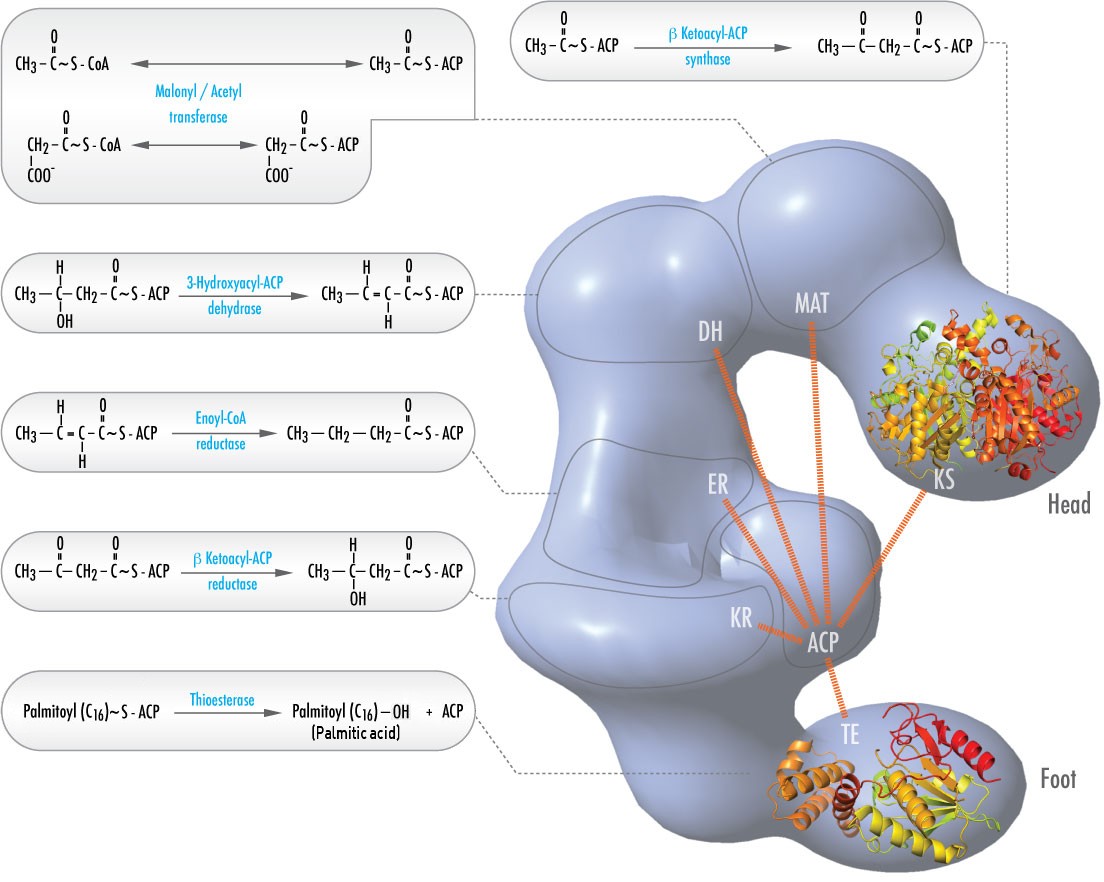
In animals, six different catalytic activities necessary for the remaining catalytic actions to fully make palmitoyl-CoA are contained in a single complex called Fatty Acid Synthase (Figure \(\PageIndex{2}\)). These include transacylases for swapping CoA with ACP on acetyl-CoA and malonyl-CoA; a synthase to catalyze addition of the two carbon unit from the three carbon malonyl-ACP in the first step of the elongation process; a reductase to reduce the ketone; a dehydrase to catalyze removal of water, and a reductase to reduce the trans double bond. In bacteria, these activities are found on separate enzymes and are not part of a complex.
Elongation of Fatty Acids
Elongation to make fatty acids longer than 16 carbons occurs in the endoplasmic reticulum and is catalyzed by enzymes described as elongases. Mitochondria also can elongate fatty acids, but their starting materials are generally shorter than 16 carbons long. The mechanisms in both environments are similar to those in the cytoplasm (a malonyl group is used to add two carbons, for example), but CoA is attached to the intermediates, not ACP. Further, whereas cytoplasmic synthesis employs the fatty acid synthase complex (Figure \(\PageIndex{2}\)), the enzymes in these organelles are separable and not part of a complex.
Desaturation of Fatty Acids
Fatty acids are synthesized in the saturated form and desaturation occurs later. Enzymes called desaturases catalyze the formation of cis double bonds in mature fatty acids. These enzymes are found in the endoplasmic reticulum. Animals are limited in the desaturated fatty acids they can make, due to an inability to catalyze reactions beyond carbons 9 and 10. Thus, humans can make oleic acid, but cannot synthesis linoleic acid or linolenic acid. Consequently, these two must be provided in the diet and are referred to as essential fatty acids.


