D. Enzyme Catalyzed Reactions in Organic Solvents
- Page ID
- 4604
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Learning Objectives
- explain from a thermodynamic and kinetic framework how enzymes retain activity in nonpolar solvents
- write chemical equations for transesterification reactions and explain their utility in the study of enzyme activity in nonpolar solvents;
- explain changes in substrate and inhibitor specificity (stereo-, regio- and chemo-) of an enzyme in a nonpolar solvent compared to water.
In the previous chapter, I showed how you could obtain information about the enzyme by changing the substrate, pH, and the enzyme. Why not change the solvent? Attempts have been made to do this for the last 100 years.
- First, water miscible solvents like ethanol and acetone were added. If the water concentration was high enough, activity remained.
- Biphasic mixtures were made in which an aqueous solution of an enzyme was emulsified in a water immiscible solvent like chloroform or ethylacetate. The substrate would partition into both phases, while the product hopefully would end up into the organic phase.
- Nearly nonaqueous solvents were used, with a few % water at less than the solubility limits of water.
- Finally, anhydrous organic solvents (0.01% water) were used. It is this later case that is most astonishing, since at first glance it is hard to believe that enzymatic activity was retained.
It is important to realize that in this last case, the enzyme is not in solution. It is rather in suspension and acts as a heterogeneous catalyst, much like palladium acts as a heterogeneous catalyst in the hydrogenation of alkenes. The suspension must be mixed vigorously and then sonicated to produce small suspended particles, so diffusion of reactants into the enzyme and out is not rate limiting. Let's explore the activity of chymotrypsin in a nonpolar solvent. Consider the following questions.
- Why aren't the enzymes inactive? Surely it must seem ridiculous that they aren't, since as we learned earlier, proteins are not that stable. A 100 amino acid protein on average is stabilized only about 10 kcal/mol over the denatured state, or the equivalent of a few H bonds. Surely the hydrophobic effect, one of the dominant contributors to protein folding and stability, would not stabilize the native structure of enzymes in nonpolar organic solvents, and the protein would denature. It doesn't however! Maybe the real question should be not whether water is necessary, but rather how much water is necessary. The enzyme can't "see" more than a monolayer or so of water around it. The data suggests that the nature of the organic solvent is very important. The most hydrophobic solvents are best in terms of their ability to maintain active enzymes! Chymotrypsin retains 104 more activity in octane than pyridine (see \(k_{cat}/K_m\) below), which is more hydrophilic than octane. The more polar the solvent, the more it can strip bound water away from the protein. If you add 1.5% water to acetone, the bound water increases from 1.2 to 2.4%, and the activity of chymotrypsin increases 1000 fold.
| Solvent | Structure | kcat/Km (M-1min-1) | relative ratio kcat/Km |
H2O bound to enzyme (%, w/w) |
|---|---|---|---|---|
| Octane |  |
63 | 15000x | 2.5 |
| Toluene | 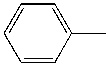 |
4.4 | 1000x | 2.3 |
| Tetrahydrofuran | 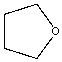 |
0.27 | 175x | 1.6 |
| Acetone | 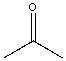 |
0.022 | 5.5x | 1.2 |
| Pyridine | 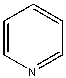 |
<0.004 | 1x (.004) | 1.0 |
- How active are enzymes in nonpolar solvents? Enzymes are often studied in model transesterification reactions. Typical reaction conditions are enzyme at 1 mg/ml, with one substrate, an ester such as N-acteyl-L-Phe-ethyl ester,at 2-12 mM, and the other substrate, an alcohol, such as n-propanol (instead of being water as in a typical hydrolysis reaction) at 0.25-1.5 M. The more concentrated alcohol replaces the alcohol (ethanol) esterified in the ester. Michaelis-Menten kinetics are followed, with biomolecular rate constants of 1010 > than without the enzyme.
- How much water do the enzymes need? 1 molecule of chymotrypsin in octane has < 50 molecules of water associated and can demonstrate activity. To form a monolayer requires about 500 water molecules. Water can be added which presumably leads to more bound water and higher activity.
- How stable are the enzymes? Denaturation requires conformational flexibility, which apparently requires water. The half-life of chymotrypsin in water at 60 °C is minutes, but in octane at 100 °C it is hours. At 20oC, the half-life in water is a few days, but in octane it is > 6 months. Remember two things contribute to stability. The protein can denature at high temperatures. Also since chymotrypsin is a protease, it can cleave itself in a autoproteolytic reaction.
| Solvent | 60oC | 100oC | 20oC |
|---|---|---|---|
| water | minutes | - | few days |
| octane | - | hours | > 6 months |
- Is the enzyme specificity changed? The net binding energy is a function of the binding energy of the substrate - the binding energy of the water, since water must be displaced from the active site on binding. In an anhydrous solvent, specificity changes must be expected. For chymotyrpsin, the driving force for binding of substrates in water is mostly hydrophobic. In water, the kcat/Km for the reaction of N-acteyl-L-Ser-esters is reduced 50,000x compared to the Phe ester. However, in octane, chymotrypsin is three times more active toward Ser esters than Phe esters.
Chymotrypsin Specificity Changes in Water and Octane Substrate \(k_{cat}/K_m\) solvent: H2O solvent: Octane N-acetyl-L-Ser-ester 1x 3x N-acetyl-L-Phe-ester 50,000x 1x
Now consider competitive inhibitors. Napthalene binds 18 times more tightly than 1-napthoic acid, but in octane, the chymotrypsin binds napthoic acid 310 times as tightly. Likewise the ratio of [kcat/Km (L isomer)]/[kcat/Km (D isomer)] of N-acetyl-D- or N-acetyl-L-Ala-chloroethyl esters is 1000-10,000 in water, but less than 10 in octane.
|
Inhibitor |
Inhibition Constant Ki (nM) |
|
|---|---|---|
| In water | In Octane | |
| Benzene | 21 | 1000 |
| Benzoic acid | 140 | 40 |
| Toluene | 12 | 1200 |
| Phenylacetic acid | 160 | 25 |
| Naphthalene | 0.4 | 1100 |
| 1-Naphthoic acid | 7.2 | 3 |
- Can new reactions be carried out in nonpolar solvents? The quick answers is yes, since reactions in aqueous solutions can be unfavorable due to low Keq's, side reactions, or insolubility of reactants. Consider lipases which cleave fatty acid esters by hydrolysis in aqueous solutions. In nonaqueous solutions, reactions such as transesterification or ammonolysis can be performed.
Enzymes are clearly active in organic solvents which appears to contradict our central concepts of protein stability. Two reasons could could explain this stability.
- It is possible that from a thermodynamic view, the enzyme is stable in organic solvents. However, as was discussed above, this is inconceivable given the delicate balance of noncovalent and hydrophobic interactions required for protein stability.
- The second reason must win the day: the protein is unable to unfold from a kinetic point of view. Conformational flexibility is required for denaturation. This must require water as the solvent.
A specific example helps illustrate the effects of different solvents on chymotrypsin activity. Dry chymotrypsin can be dissolved in DMSO, a water miscible solvent. In this solvent it is completely and irreversibly denatured. If it is now diluted 50X with acetone with 3% water, no activity is observed. (In the final dilution, the concentrations of solvents are 98% acetone, 2.9% water, and 2% DMSO.) However, if dry chymotrypsin was added to a mixture of 98% acetone, 2.9% water, and 2% DMSO, the enzyme is very active. We end up with the same final solvent state, but in the first case the enzyme has no activity while in the second case it retains activity.
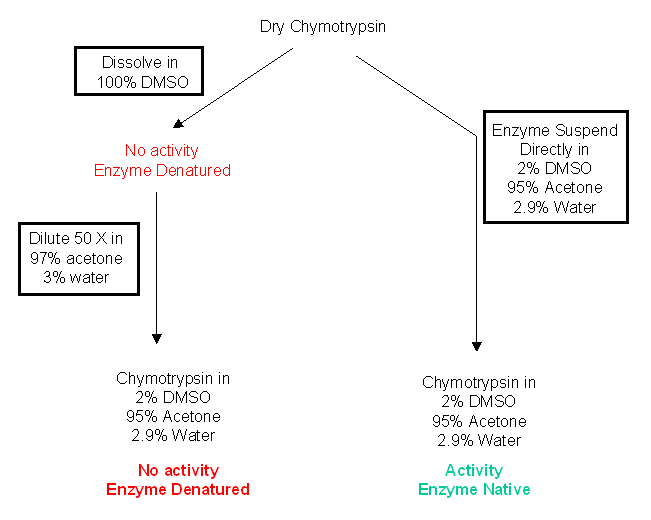
Dry enzymes added to a concentrated water-miscible organic solvent (like DMSO) will dissolve and surely denature, but will retain activity when added to a concentrated water-immiscible solvent (like octane), in which the enzyme will not dissolve but stay in suspension.
It appears the enzymes have very restricted conformational mobility in nonpolar solvents. By lyophilizing (freeze-drying) the enzyme against a specific ligand, a given conformation of a protein can be trapped or literally imprinted onto the enzyme. For example, if the enzyme is dialyzed against a competitive inhibitor (which can be extracted by the organic solvent), freeze-dried to remove water, and then added to a nonpolar solvent, the enzyme activity of the "imprinted" enzyme in nonpolar solvents is as much as 100x as great as when no inhibitor was present during the dialysis. If chymotrypsin is lyophilized from solutions of different pHs, the resulting curve of V/Km for ester hydrolysis in octane is bell-shaped with the initial rise in activity reaching half-maximum activity at a pH of around 6.0 and a fall in activity reaching half-maximum at pH of approximately 9.
Use of enzymes in organic solvent allows new routes to organic synthesis. Enzymes, which are so useful in synthetic reactions, are:
- stereoselective - can differentiate between enantiomers and between prochiral substrates
- regioselective - can differentiate between identical functional groups in a single substrate
- chemoselective - can differentiate between different functional groups in a substrate (such as between a hydroxyl group and an amine for an acylation reaction)
Enzyme in anhydrous organic solvents are useful (from a synthetic point) not only since new types of reactions can be catalyzed (such as transesterification, ammonolysis, thiolysis) but also because the stereoselectivity, regioselectivity, and chemoselectivity of the enzyme often changes from activities of the enzyme in water.
Organic Reactions in Water?
Organic reactions are usually conducted in organic solvents, since many organic molecules react with water, and the reagents and products are usually not soluble in water. In a manner analogous to using an enzyme as a heterogeneous catalyst in nonpolar solvent, Sharpless is pioneering a technique to conduct organic reactions in water. They (Narayan et al.) have shown that many unimolecular and bimolecular reactions occur faster in water than in organic solvents. As in enzyme catalysis in nonpolar solvent, the reactions must be mixed vigorously to disperse reactants in micro-drops (a suspension) in water, greatly increasing the surface area that might allow water to act on transition states or intermediates to stabilize them through hydrogen bonding. They called these reactions "on water" reactions since reactants usually float on water. They have performed cycloadditions, alkene reactions, Claisen rearrangments, and nucleophilic substitution reactions using this process. One cycloaddition reaction went to completion in ten minutes at room temperature, compared to 18 hours in methanol and 120 in toluene. Adding nonpolar solvent at certain times greatly increased the rate of the reaction.
Recent References
- Klijn, J and Engberts, J. Organic chemistry: Fast reactions 'on water'. Nature 435, 746-747 (9 June 2005) | doi:10.1038/435746a
- Narayan, S. et al. Angew. Chem. Intl. Edn 44, 3275 (2005)
- Klibanov. Improving enzymes by using them in organic solvents. Nature. 409. pg 241 (2001)


