W2018_Bis2A_Lecture09_reading
- Page ID
- 10599
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Membranes
Plasma membranes enclose and define the borders between the inside and the outside of cells. They are typically composed of dynamic bilayers of phospholipids into which various other lipid soluble molecules and proteins have also been embedded. These bilayers are asymmetric—the outer leaf being different than the inner leaf in lipid composition and in the proteins and carbohydrates that are displayed to either the inside or outside of the cell. Various factors influence the fluidity, permeability, and various other physical properties of the membrane. These include the temperature, the configuration of the fatty acid tails (some kinked by double bonds), the presence of sterols (i.e., cholesterol) embedded in the membrane, and the mosaic nature of the proteins embedded within it. The cell membrane has selectivity; it allows only some substances through while excluding others. In addition, the plasma membrane must, in some cases, be flexible enough to allow certain cells, such as amoebae, to change shape and direction as they move through the environment, hunting smaller, single-celled organisms.
Amoebae Hunting Video
Cellular membranes
A subgoal in our "build-a-cell" design challenge is to create a boundary that separates the "inside" of the cell from the environment "outside". This boundary needs to serve multiple functions that include:
- Act as a barrier by blocking some compounds from moving in and out of the cell.
- Be selectively permeable in order to transport specific compounds into and out of the cell.
- Receive, sense, and transmit signals from the environment to inside of the cell.
- Project "self" to others by communicating identity to other nearby cells.

Figure 1. The diameter of a typical balloon is 25cm and the thickness of the plastic of the balloon of around 0.25mm. This is a 1000X difference. A typical eukaryotic cell will have a cell diameter of about 50µm and a cell membrane thickness of 5nm. This is a 10,000X difference.
Note: possible discussion
The ratio of membrane thickness compared to the size of an average eukaryotic cell is much greater compared to that of a balloon stretched with air. To think that the boundary between life and nonlife is so small, and seemingly fragile, more so than a balloon, suggests that structurally the membrane must be relatively stable. Discuss why cellular membranes are stable. You will need to pull from information we have already covered in this class.
Fluid mosaic model
The existence of the plasma membrane was identified in the 1890s, and its chemical components were identified in 1915. The principal components identified at that time were lipids and proteins. The first widely accepted model of the plasma membrane’s structure was proposed in 1935 by Hugh Davson and James Danielli; it was based on the “railroad track” appearance of the plasma membrane in early electron micrographs. They theorized that the structure of the plasma membrane resembles a sandwich, with protein being analogous to the bread, and lipids being analogous to the filling. In the 1950s, advances in microscopy, notably transmission electron microscopy (TEM), allowed researchers to see that the core of the plasma membrane consisted of a double, rather than a single, layer. A new model that better explains both the microscopic observations and the function of that plasma membrane was proposed by S.J. Singer and Garth L. Nicolson in 1972.
The explanation proposed by Singer and Nicolson is called the fluid mosaic model. The model has evolved somewhat over time, but it still best accounts for the structure and functions of the plasma membrane as we now understand them. The fluid mosaic model describes the structure of the plasma membrane as a mosaic of components—including phospholipids, cholesterol, proteins, and carbohydrates—that gives the membrane a fluid character. Plasma membranes range from 5 to 10 nm in thickness. For comparison, human red blood cells, visible via light microscopy, are approximately 8 µm wide, or approximately 1,000 times wider than a plasma membrane.

Figure 2. The fluid mosaic model of the plasma membrane describes the plasma membrane as a fluid combination of phospholipids, cholesterol, and proteins. Carbohydrates attached to lipids (glycolipids) and to proteins (glycoproteins) extend from the outward-facing surface of the membrane.
The principal components of a plasma membrane are lipids (phospholipids and cholesterol), proteins, and carbohydrates. The proportions of proteins, lipids, and carbohydrates in the plasma membrane vary with organism and cell type, but for a typical human cell, proteins account for about 50 percent of the composition by mass, lipids (of all types) account for about 40 percent of the composition by mass, and carbohydrates account for the remaining 10 percent of the composition by mass. However, the concentration of proteins and lipids varies with different cell membranes. For example, myelin, an outgrowth of the membrane of specialized cells, insulates the axons of the peripheral nerves, contains only 18 percent protein and 76 percent lipid. The mitochondrial inner membrane contains 76 percent protein and only 24 percent lipid. The plasma membrane of human red blood cells is 30 percent lipid. Carbohydrates are present only on the exterior surface of the plasma membrane and are attached to proteins, forming glycoproteins, or to lipids, forming glycolipids.
Phospholipids
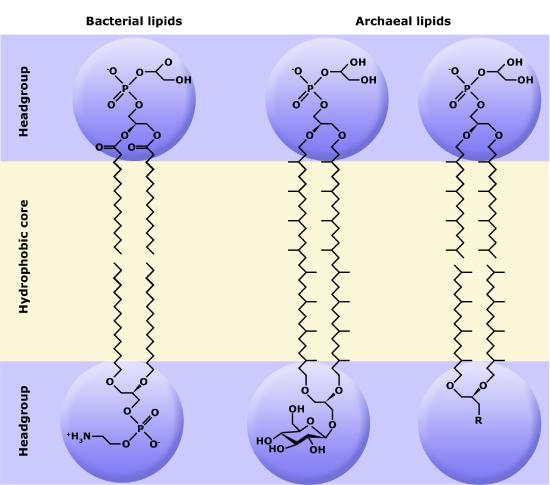
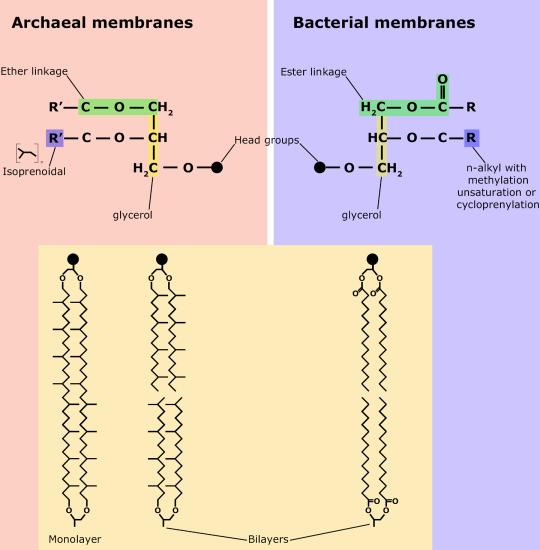
Phospholipids are major constituents of the cell membrane, the outermost layer of cells. Like fats, they are composed of fatty acid chains attached to a polar head group. Specifically, there are two fatty acid tails and a phosphate group as the polar head group. The phospholipid is an amphipathic molecule, meaning it has a hydrophobic part and a hydrophilic part. The fatty acid chains are hydrophobic and cannot interact with water, whereas the phosphate-containing head group is hydrophilic and interacts with water.
Note
Make sure to note in Figure 3 that the phosphate group has an R group linked to one of the oxygen atoms. R is a variable commonly used in these types of diagrams to indicate that some other atom or molecule is bound at that position. That part of the molecule can be different in different phospholipids—and will impart some different chemistry to the whole molecule. At the moment, however, you are responsible for being able to recognize this type of molecule (no matter what the R group is) because of the common core elements—the glycerol backbone, the phosphate group, and the two hydrocarbon tails.

Figure 3. A phospholipid is a molecule with two fatty acids and a modified phosphate group attached to a glycerol backbone. The phosphate may be modified by the addition of charged or polar chemical groups. Several chemical R groups may modify the phosphate. Choline, serine, and ethanolamine are shown here. These attach to the phosphate group at the position labeled R via their hydroxyl groups.
Attribution: Marc T. Facciotti (own work)
A phospholipid bilayer forms as the basic structure of the cell membrane. The fatty acid tails of phospholipids face inside, away from water, whereas the phosphate group faces outside, hydrogen bonding with water. Phospholipids are responsible for the dynamic nature of the plasma membrane.

Figure 4. In the presence of water, some phospholipids will spontaneously arrange themselves into a micelle. The lipids will be arranged such that their polar groups will be on the outside of the micelle, and the nonpolar tails will be on the inside. A lipid bilayer can also form, a two layered sheet only a few nanometers thick. The lipid bilayer consists of two layers of phospholipids organized in a way that all the hydrophobic tails align side by side in the center of the bilayer and are surrounded by the hydrophilic head groups.
Source: Created by Erin Easlon (own work)
Note: possible discussion
Above it says that if you were to take some pure phospholipids and drop them into water that some if it would spontaneously (on its own) form into micelles. This sounds a lot like something that could be described by an energy story. Go back to the energy story rubric and try to start creating an energy story for this process—I expect that the steps involving the description of energy might be difficult at this point (we'll come back to that later) but you should be able to do at least the first three steps. You can constructively critique (politely) each other's work to create an optimized story.
Note: possible discussion
Note that the phospholipid depicted above has an R group linked to the phosphate group. Recall that this designation is generic—these can be different than the R groups on amino acids. What might be a benefit/purpose of "functionalizing" or "decorating" different lipids with different R groups? Think of the functional requirements for membranes stipulated above.
Membrane proteins
Proteins make up the second major component of plasma membranes. Integral membrane proteins are, as their name suggests, integrated completely into the membrane structure, and their hydrophobic membrane-spanning regions interact with the hydrophobic region of the the phospholipid bilayer. Single-pass integral membrane proteins usually have a hydrophobic transmembrane segment that consists of 20–25 amino acids. Some span only part of the membrane—associating with a single layer—while others stretch from one side of the membrane to the other, and are exposed on either side. This type of protein has a hydrophilic region or regions, and one or several mildly hydrophobic regions. This arrangement of regions of the protein tends to orient the protein alongside the phospholipids, with the hydrophobic region of the protein adjacent to the tails of the phospholipids and the hydrophilic region or regions of the protein protruding from the membrane and in contact with the cytosol or extracellular fluid.
Peripheral proteins are found on either the exterior or interior surfaces of membranes; and weakly or temporarily associated with the membranes. They can interact with either integral membrane proteins or simply interact weakly with the phospholipids within the membrane.

Figure 5. Integral membranes proteins may have one or more α-helices (pink cylinders) that span the membrane (examples 1 and 2), or they may have β-sheets (blue rectangles) that span the membrane (example 3). (credit: “Foobar”/Wikimedia Commons)
Carbohydrates
Carbohydrates are the third major component of plasma membranes. They are always found on the exterior surface of cells and are bound either to proteins (forming glycoproteins) or to lipids (forming glycolipids). These carbohydrate chains may consist of 2–60 monosaccharide units and can be either straight or branched. Along with peripheral proteins, carbohydrates form specialized sites on the cell surface that allow cells to recognize each other (one of the core functional requirements noted above in "cellular membranes").
Membrane fluidity
The mosaic characteristic of the membrane, described in the fluid mosaic model, helps to illustrate its nature. The integral proteins and lipids exist in the membrane as separate molecules and they "float" in the membrane, moving somewhat with respect to one another. The membrane is not like a balloon, however, in that can expand and contract dramatically; rather, it is fairly rigid and can burst if penetrated or if a cell takes in too much water. However, because of its mosaic nature, a very fine needle can easily penetrate a plasma membrane without causing it to burst, and the membrane will flow and self-seal when the needle is extracted.
The mosaic characteristics of the membrane explain some but not all of its fluidity. There are two other factors that help maintain this fluid characteristic. One factor is the nature of the phospholipids themselves. In their saturated form, the fatty acids in phospholipid tails are saturated with hydrogen atoms. There are no double bonds between adjacent carbon atoms. This results in tails that are relatively straight. By contrast, unsaturated fatty acids do not have a full complement of hydrogen atoms on their fatty acid tails, and therefore contain some double bonds between adjacent carbon atoms; a double bond results in a bend in the string of carbons of approximately 30 degrees.

Figure 6. Any given cell membrane will be composed of a combination of saturated and unsaturated phospholipids. The ratio of the two will influence the permeability and fluidity of the membrane. A membrane composed of completely saturated lipids will be dense and less fluid, and a membrane composed of completely unsaturated lipids will be very loose and very fluid.
Note: possible discussion
Organisms can be found living in extreme temperature conditions. Both in extreme cold or extreme heat. What types of differences would you expect to see in the lipid composition of organisms that live at these extremes?
Saturated fatty acids, with straight tails, are compressed by decreasing temperatures, and they will press in on each other, making a dense and fairly rigid membrane. When unsaturated fatty acids are compressed, the “kinked” tails elbow adjacent phospholipid molecules away, maintaining some space between the phospholipid molecules. This “elbow room” helps to maintain fluidity in the membrane at temperatures at which membranes with high concentrations of saturated fatty acid tails would “freeze” or solidify. The relative fluidity of the membrane is particularly important in a cold environment. Many organisms (fish are one example) are capable of adapting to cold environments by changing the proportion of unsaturated fatty acids in their membranes in response to the lowering of the temperature.
Cholesterol
Animals have an additional membrane constituent that assists in maintaining fluidity. Cholesterol, which lies alongside the phospholipids in the membrane, tends to dampen the effects of temperature on the membrane. Thus, this lipid functions as a "fluidity buffer", preventing lower temperatures from inhibiting fluidity and preventing increased temperatures from increasing fluidity too much. Thus, cholesterol extends, in both directions, the range of temperature in which the membrane is appropriately fluid and consequently functional. Cholesterol also serves other functions, such as organizing clusters of transmembrane proteins into lipid rafts.

Figure 7. Cholesterol fits between the phospholipid groups within the membrane.
Review of the components of the membrane
| The components and functions of the plasma membrane | |
|---|---|
| Component | Location |
| Phospholipid | Main fabric of the membrane |
| Cholesterol | Between phospholipids and between the two phospholipid layers of animal cells |
| Integral proteins (e.g., integrins) | Embedded within the phospholipid layer(s); may or may not penetrate through both layers |
| Peripheral proteins | On the inner or outer surface of the phospholipid bilayer; not embedded within the phospholipids |
| Carbohydrates (components of glycoproteins and glycolipids) | Generally attached to proteins on the outside membrane layer |
Transport across the membrane
Design challenge problem and subproblems
General Problem: The cell membrane must simultaneously act as a barrier between "IN" and "OUT" and control specifically which substances enter and leave the cell and how quickly and efficiently they do so.
Subproblems: The chemical properties of molecules that must enter and leave the cell are highly variable. Some subproblems associated with this are: (a) Large and small molecules or collections of molecules must be able to pass across the membrane. (b) Both hydrophobic and hydrophilic substances must have access to transport. (c) Substances must be able to cross the membrane with and against concentration gradients. (d) Some molecules look very similar (e.g. Na+ and K+) but transport mechanisms must still be able to distinguish between them.
Energy story perspective
Transport across a membrane can be considered from an energy story perspective; it is a process after all. For instance, at the beginning of the process a generic substance X may be either on the inside or outside of the cell. At the end of the process, the substance will be on the opposite side from which it started.
e.g. X(in) ---> X(out),
where in and out refer to inside the cell and outside the cell, respectively.
At the beginning the matter in the system might be a very complicated collection of molecules inside and outside of the cell but with one molecule of X more inside the cell than out. At the end, there is one more molecule of X on the outside of the cell and one less on the inside. The energy in the system at the beginning is stored largely in the molecular structures and their motions and in electrical and chemical concentration imbalances across the cell membrane. The transport of X out of the cell will not change the energies of the molecular structures significantly but it will change the energy associated with the imbalance of concentration and or charge across the membrane. That is the transport will, like all other reactions, be either exergonic or endergonic. Finally, some mechanism or sets of mechanisms of transport will need to be described.
Selective permeability
One of the great wonders of the cell membrane is its ability to regulate the concentration of substances inside the cell. These substances include: ions such as Ca2+, Na+, K+, and Cl–; nutrients including sugars, fatty acids, and amino acids; and waste products, particularly carbon dioxide (CO2), which must leave the cell.
The membrane’s lipid bilayer structure provides the first level of control. The phospholipids are tightly packed, and the membrane has a hydrophobic interior. This structure alone creates what is known as a selectively permeable barrier, one that only allows substances meeting certain physical criteria to pass through it. In the case of the cell membrane, only relatively small, nonpolar materials can move through the lipid bilayer at biologically relevant rates (remember, the lipid tails of the membrane are nonpolar).
Selective permeability of the cell membrane refers to its ability to differentiate between different types of molecules, only allowing some molecules through while blocking others. Some of this selective property stems from the intrinsic diffusion rates for different molecules across a membrane. A second factor affecting the relative rates of movement of various substances across a biological membrane is activity of various protein-based membrane transporters, both passive and active, that will be discussed in more detail in subsequent sections. First, we take on the notion of intrinsic rates of diffusion across the membrane.
Relative permeability
The fact that different substances might cross a biological membrane at different rates should be relatively intuitive. There are differences in the mosaic composition of membranes in biology and differences in the sizes, flexibility, and chemical properties of molecules so it stands to reason that the permeability rates vary. It is a complicated landscape. The permeability of a substance across a biological membrane can be measured experimentally and the rate of movement across a membrane can be reported in what are known as membrane permeability coefficients.
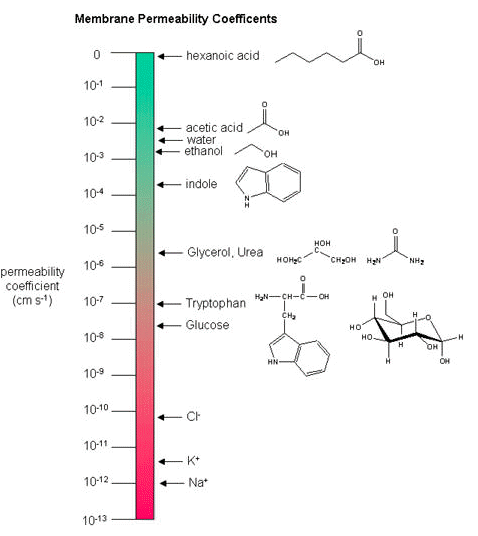
Membrane permeability coefficients
Below, a variety of compounds are plotted with respect to their membrane permeability coefficients (MPC) as measured against a simple biochemical approximation of a real biological membrane. The reported permeability coefficient for this system is the rate at which simple diffusion through a membrane occurs and is reported in units of centimeters per second (cm/s). The permeability coefficient is proportional to the partition coefficient and is inversely proportional to the membrane thickness.
It is important that you are able to read and interpret the diagram below. The larger the coefficient, the more permeable the membrane is to the solute. For example, hexanoic acid is very permeable, a MPC of 0.9; acetic acid, water, and ethanol have MPCs between 0.01 and 0.001, and they are less permeable than hexanoic acid. Where as ions, such as sodium (Na+), have an MPC of 10-12, and cross the membrane at a comparatively slow rate.
While there are certain trends or chemical properties that can be roughly associated with different compound permeability (small thing go through "fast", big things "slowly", charged things not at all etc.), we caution against over-generalizing. The molecular determinants of membrane permeability are complicated and involve numerous factors including: the specific composition of the membrane, temperature, ionic composition, hydration; the chemical properties of the solute; the potential chemical interactions between the solute in solution and in the membrane; the dielectric properties of materials; and the energy trade-offs associated with moving substances into and out of various environments. So, in this class, rather than try to apply "rules" and try to develop too many arbitrary "cut-offs", we will strive to develop a general sense of some properties that can influence permeability and leave the assignment of absolute permeability to experimentally reported rates. In addition, we will also try to minimize the use of vocabulary that depends on a frame of reference. For instance, saying that compound A diffuses "quickly" or "slowly" across a bilayer only means something if the terms "quickly" or "slowly" are numerically defined or the biological context is understood.
Energetics of transport
All substances that move through the membrane do so by one of two general methods, which are categorized based on whether or not the transport process is exergonic or endergonic. Passive transport is the exergonic movement of substances across the membrane. In contrast, active transport is the endergonic movement of substances across the membrane that is coupled to an exergonic reaction.
Passive transport
Passive transport does not require the cell to expend energy. In passive transport, substances move from an area of higher concentration to an area of lower concentration, down their concentration gradient. Depending on the chemical nature of the substance, different processes may be associated with passive transport.
Diffusion
Diffusion is a passive process of transport. A single substance tends to move from an area of high concentration to an area of low concentration until the concentration is equal across a space. You are familiar with diffusion of substances through the air. For example, think about someone opening a bottle of ammonia in a room filled with people. The ammonia gas is at its highest concentration in the bottle; its lowest concentration is at the edges of the room. The ammonia vapor will diffuse, or spread away, from the bottle; gradually, more and more people will smell the ammonia as it spreads. Materials move within the cell’s cytosol by diffusion, and certain materials move through the plasma membrane by diffusion.
Factors that affect diffusion
If unconstrained, molecules will move through and explore space randomly at a rate that depends on their size, their shape, their environment, and their thermal energy. This type of movement underlies the diffusive movement of molecules through whatever medium they are in. The absence of a concentration gradient does not mean that this movement will stop, just that there may be no net movement of the number of molecules from one area to another, a condition known as dynamic equilibrium.
Factors influencing diffusion include:
- Extent of the concentration gradient: The greater the difference in concentration, the more rapid the diffusion. The closer the distribution of the material gets to equilibrium, the slower the rate of diffusion becomes.
- Shape, size and mass of the molecules diffusing: Large and heavier molecules move more slowly; therefore, they diffuse more slowly. The reverse is typically true for smaller, lighter molecules.
- Temperature: Higher temperatures increase the energy and therefore the movement of the molecules, increasing the rate of diffusion. Lower temperatures decrease the energy of the molecules, thus decreasing the rate of diffusion.
- Solvent density: As the density of a solvent increases, the rate of diffusion decreases. The molecules slow down because they have a more difficult time getting through the denser medium. If the medium is less dense, rates of diffusion increase. Since cells primarily use diffusion to move materials within the cytoplasm, any increase in the cytoplasm’s density will decrease the rate at which materials move in the cytoplasm.
- Solubility: As discussed earlier, nonpolar or lipid-soluble materials pass through plasma membranes more easily than polar materials, allowing a faster rate of diffusion.
- Surface area and thickness of the plasma membrane: Increased surface area increases the rate of diffusion, whereas a thicker membrane reduces it.
- Distance traveled: The greater the distance that a substance must travel, the slower the rate of diffusion. This places an upper limitation on cell size. A large, spherical cell will die because nutrients or waste cannot reach or leave the center of the cell, respectively. Therefore, cells must either be small in size, as in the case of many prokaryotes, or be flattened, as with many single-celled eukaryotes.
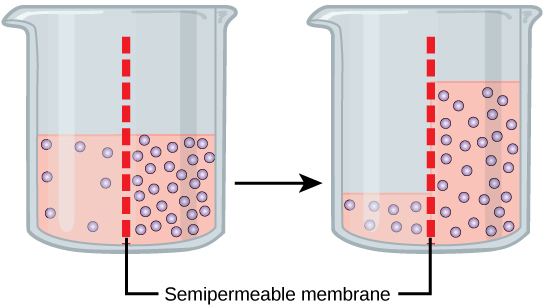
Osmosis
Osmosis is the movement of water through a semipermeable membrane according to the concentration gradient of water across the membrane, which is inversely proportional to the concentration of solutes. While diffusion transports material across membranes and within cells, osmosis transports only water across a membrane and the membrane limits the diffusion of solutes in the water. Not surprisingly, the aquaporins that facilitate water movement play a large role in osmosis, most prominently in red blood cells and the membranes of kidney tubules.
Mechanism
Osmosis is a special case of diffusion. Water, like other substances, moves from an area of high concentration to one of low concentration. An obvious question is what makes water move at all? Imagine a beaker with a semipermeable membrane separating the two sides or halves. On both sides of the membrane the water level is the same, but there are different concentrations of a dissolved substance, or solute, that cannot cross the membrane (otherwise the concentrations on each side would be balanced by the solute crossing the membrane). If the volume of the solution on both sides of the membrane is the same, but the concentrations of solute are different, then there are different amounts of water, the solvent, on either side of the membrane.
To illustrate this, imagine two full glasses of water. One has a single teaspoon of sugar in it, whereas the second one contains one-quarter cup of sugar. If the total volume of the solutions in both cups is the same, which cup contains more water? Because the large amount of sugar in the second cup takes up much more space than the teaspoon of sugar in the first cup, the first cup has more water in it.
Returning to the beaker example, recall that it has a mixture of solutes on either side of the membrane. A principle of diffusion is that the molecules move around and will spread evenly throughout the medium if they can. However, only the material capable of getting through the membrane will diffuse through it. In this example, the solute cannot diffuse through the membrane, but the water can. Water has a concentration gradient in this system. Thus, water will diffuse down its concentration gradient, crossing the membrane to the side where it is less concentrated. This diffusion of water through the membrane—osmosis—will continue until the concentration gradient of water goes to zero or until the hydrostatic pressure of the water balances the osmotic pressure. Osmosis proceeds constantly in living systems.
Tonicity
Tonicity describes how an extracellular solution can change the volume of a cell by affecting osmosis. A solution's tonicity often directly correlates with the osmolarity of the solution. Osmolarity describes the total solute concentration of the solution. A solution with low osmolarity has a greater number of water molecules relative to the number of solute particles; a solution with high osmolarity has fewer water molecules with respect to solute particles. In a situation in which solutions of two different osmolarities are separated by a membrane permeable to water, though not to the solute, water will move from the side of the membrane with lower osmolarity (and more water) to the side with higher osmolarity (and less water). This effect makes sense if you remember that the solute cannot move across the membrane, and thus the only component in the system that can move—the water—moves along its own concentration gradient. An important distinction that concerns living systems is that osmolarity measures the number of particles (which may be molecules) in a solution. Therefore, a solution that is cloudy with cells may have a lower osmolarity than a solution that is clear if the second solution contains more dissolved molecules than there are cells.
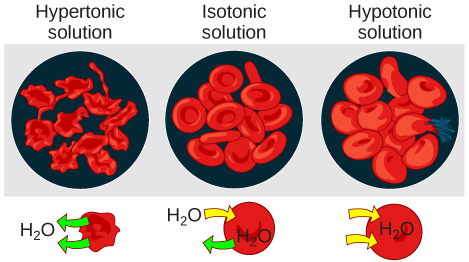
Hypotonic solutions
Three terms—hypotonic, isotonic, and hypertonic—are used to relate the osmolarity of a cell to the osmolarity of the extracellular fluid that contains the cells. In a hypotonic situation, the extracellular fluid has lower osmolarity than the fluid inside the cell, and water enters the cell (in living systems, the point of reference is always the cytoplasm, so the prefix hypo- means that the extracellular fluid has a lower concentration of solutes, or a lower osmolarity, than the cell cytoplasm). It also means that the extracellular fluid has a higher concentration of water in the solution than does the cell. In this situation, water will follow its concentration gradient and enter the cell.
Hypertonic solutions
As for a hypertonic solution, the prefix hyper- refers to the extracellular fluid having a higher osmolarity than the cell’s cytoplasm; therefore, the fluid contains less water than the cell does. Because the cell has a relatively higher concentration of water, water will leave the cell.
Isotonic solutions
In an isotonic solution, the extracellular fluid has the same osmolarity as the cell. If the osmolarity of the cell matches that of the extracellular fluid, there will be no net movement of water into or out of the cell, although water will still move in and out. Blood cells and plant cells in hypertonic, isotonic, and hypotonic solutions take on characteristic appearances.
Connection
A doctor injects a patient with what the doctor thinks is an isotonic saline solution. The patient dies, and an autopsy reveals that many red blood cells have been destroyed. Do you think the solution the doctor injected was really isotonic?
Link to learning
For a video illustrating the process of diffusion in solutions, visit this site.
Tonicity in living systems
In a hypotonic environment, water enters a cell, and the cell swells. In an isotonic condition, the relative concentrations of solute and solvent are equal on both sides of the membrane. There is no net water movement; therefore, there is no change in the size of the cell. In a hypertonic solution, water leaves a cell and the cell shrinks. If either the hypo- or hyper- condition goes to excess, the cell’s functions become compromised, and the cell may be destroyed.
A red blood cell will burst, or lyse, when it swells beyond the plasma membrane’s capability to expand. Remember, the membrane resembles a mosaic, with discrete spaces between the molecules composing it. If the cell swells, and the spaces between the lipids and proteins become too large, and the cell will break apart.
In contrast, when excessive amounts of water leave a red blood cell, the cell shrinks, or crenates. This has the effect of concentrating the solutes left in the cell, making the cytosol denser and interfering with diffusion within the cell. The cell’s ability to function will be compromised and may also result in the death of the cell.
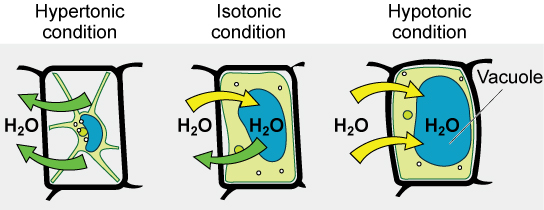
Various living things have ways of controlling the effects of osmosis—a mechanism called osmoregulation. Some organisms, such as plants, fungi, bacteria, and some protists, have cell walls that surround the plasma membrane and prevent cell lysis in a hypotonic solution. The plasma membrane can only expand to the limit of the cell wall, so the cell will not lyse. In fact, the cytoplasm in plants is always slightly hypertonic to the cellular environment, and water will always enter a cell if water is available. This inflow of water produces turgor pressure, which stiffens the cell walls of the plant. In nonwoody plants, turgor pressure supports the plant. Conversely, if the plant is not watered, the extracellular fluid will become hypertonic, causing water to leave the cell. In this condition, the cell does not shrink because the cell wall is not flexible. However, the cell membrane detaches from the wall and constricts the cytoplasm. This is called plasmolysis. Plants lose turgor pressure in this condition and wilt.
Tonicity is a concern for all living things. For example, paramecia and amoebas, which are protists that lack cell walls, have contractile vacuoles. This vesicle collects excess water from the cell and pumps it out, keeping the cell from bursting as it takes on water from its environment.
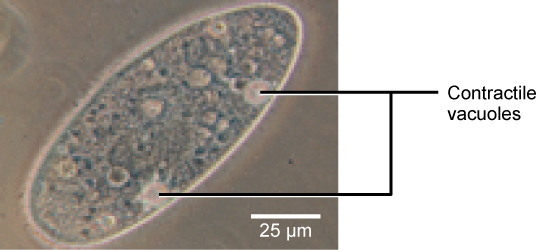
Figure 12. A paramecium’s contractile vacuole, here visualized using bright field light microscopy at 480x magnification, continuously pumps water out of the organism’s body to keep it from bursting in a hypotonic medium. (credit: modification of work by NIH; scale-bar data from Matt Russell)
Many marine invertebrates have internal salt levels matched to their environments, making them isotonic with the water in which they live. Fish, however, must spend approximately five percent of their metabolic energy maintaining osmotic homeostasis. Freshwater fish live in an environment that is hypotonic to their cells. These fish actively take in salt through their gills and excrete diluted urine to rid themselves of excess water. Saltwater fish live in the reverse environment, which is hypertonic to their cells, and they secrete salt through their gills and excrete highly concentrated urine.
In vertebrates, the kidneys regulate the amount of water in the body. Osmoreceptors are specialized cells in the brain that monitor the concentration of solutes in the blood. If the levels of solutes increase beyond a certain range, a hormone is released that retards water loss through the kidney and dilutes the blood to safer levels. Animals also have high concentrations of albumin, which is produced by the liver, in their blood. This protein is too large to pass easily through plasma membranes and is a major factor in controlling the osmotic pressures applied to tissues.