1.17: Gastrointestinal Anatomy and Digestion
- Page ID
- 59163
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)Objectives:
At the end of this lab, you will be able to…
1. Correctly use anatomical terminology to identify the organs and tissues involved with the overall digestion of materials being consumed
2. Explain why mechanical digestion only occurs in the mouth by teeth and muscle activity
3. Describe process of chemical digestion and reason for process requiring enzymes
4. Determine the role of the tissue and /organs of the gastrointestinal system for overall regulation of homeostasis
Pre-Lab Exercises:
After reading through the lab activities prior to lab, complete the following before you start your lab.
1. The sections of the alimentary canal are the: .
2. The pattern of teeth we have is considered to be and includes incisors, canines (cuspid), pre-molars (bicuspid) and molars.
3. Color the images for use as a reference for identifying the organs.
4. Highlight key steps to the chemical digestion of carbohydrates and proteins
5. How long will chemical digestion of carbohydrates last?
6. How long will chemical digestion of proteins last?
Materials:
- Torso, Head and Mouth Models
- Stickers
- Felt Pens
- Colored Pencils
- Chemical Digestion Kits
The gastrointestinal system has been refined to transfer organic nutrients, minerals and water from the external environment into the internal environment. This process is specialized within the various organs and tissues within the system with a four-stage process utilized in order to accomplish the general function based on the interaction between three distinct segments of organs. The entire process takes places within an elongated opening within the body that while contained with the body, encapsulates a lumen (alimentary canal) that is can be thought of as being external to the body. The length (28-feet) and histology (high level of surface area) ensures complete digestion and absorption of all materials that enter the lumen within approximately 24-hours following initial digestion in the mouth.
The first-stage is the mechanical digestion, taking the large bolus and breaking it into smaller bolus prior to entering the stomach, and takes place within the first segment of the system (the oral opening) via mastication (use of teeth and muscles of jaw). The end of mastication results in swallowing and the initiation of peristalsis, the coordinated contraction of muscles (circular and longitudinal smooth muscles) of the alimentary canal to propel food materials from the mouth to the anus in roughly 24-hours.
The second-stage is the transportation and initial chemical digestion of the food material. This stage occurs in the second segment (esophagus and stomach) where the smaller food boluses that were swallowed is then exposed to a low pH (acidic) solution liquefies the material. This liquefaction is necessary for the third-stage in the next segment (small intestines) for the further chemical digestion by specific enzymes into the individual components (e.g., fats, carbohydrates, amino acids, nucleic acids, inorganic and organic salts). The fourth-stage takes place in the small and large intestines and is responsible for the absorption of the individual food components that have been digested so far within the system. This final segment is also responsible for the reabsorption of water and removal of undigested and nondigestible materials out of the system.
The anatomy of the alimentary canal (esophagus, stomach, small and large intestines) and accessory organs (liver, gall bladder and pancreas) of the system function to maximize the amount that can be digested and absorbed based on blood flow through the tissues. Most organs will function based solely on the ingestion of materials and are not regulated to alter the amount of any individual type of material that can be absorbed. Any material that is not digested (non-digestible) or incompletely digested will not be absorbed and will be removed as fecal matter via defecation, along with any excretions in the lumen. Of the material in the lumen, only 1% will be excreted in the form of fecal matter. The rate at which materials move through the system is based on the regulatory that the material is eaten, the amount that is eaten and the level of hydration. For most meals, materials that will be digested, absorbed and fecal waste removed within 24-hours.
The system is also involved with the regulation of when and how much we eat via the coordinated signals from hormones (LEPTIN, ghrelin, pancreatic hormones (PYY, insulin, glucagon), neurohormones (NPY, POMC, endocabinoids), gut hormones (gastrin, secretin, CCK), and AMPkinase) along with stretch receptors within the alimentary canal that either initiates the pull-to (“I need to eat”) or push-away (“I’m full”) feeding responses through signals from the hypothalamus.
**Set-up Chemical Digestion in Activity 3 before completing the rest of the lab**
Activity 1: Organs of Digestion
Procedures:
1. Obtain torso and head models, stickers, felt pens
2. Write names of organs and structures that you are responsible for knowing and identifying on the stickers
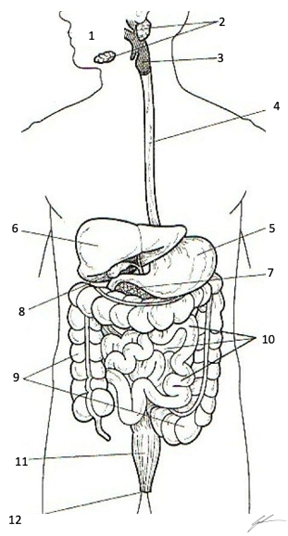
Esophagus, Stomach, Liver, Pancreas, Gallbladder, Duodenum, Small Intestine, Large Intestine, Rectum
|
Organs and Structures of the Digestive System 1 Oral Opening (Mouth) 2 Salivary Glands 3 Oropharynx with Epiglottis and Glottis 4 Esophagus 5 Stomach 6 Liver 7 Pancreas 8 Gallbladder 9 Colon (Large Intestine) 10 Duodenum, Jejunum, Ileum (Small Intestine) 11 Rectum 12 Anus Color each structure, or organ with a different color to assist with identification |
3. Select a “team leader” and use the colored images and reference materials to take turns labeling the torso model
a. As structures are labeled, indicate a function of the organ or structure for digestion.
|
Structure |
Function |
|
Teeth |
|
|
Salivary Glands |
|
|
Glottis and Epiglottis |
|
|
Esophagus |
|
|
Stomach |
|
|
Duodenum |
|
|
Small Intestine |
|
|
Liver and Gallbladder |
|
|
Pancreas |
|
|
Large Intestine |
|
|
Rectum |
4. Have your instructor check your progress and move to activity 2.
Activity 2: Anatomy of the Oral Opening and Teeth
Procedures:
1. Obtain torso and sagittal head models, stickers, felt pens
2. Write names of structures of the mouth that you are responsible for knowing and identifying on the stickers
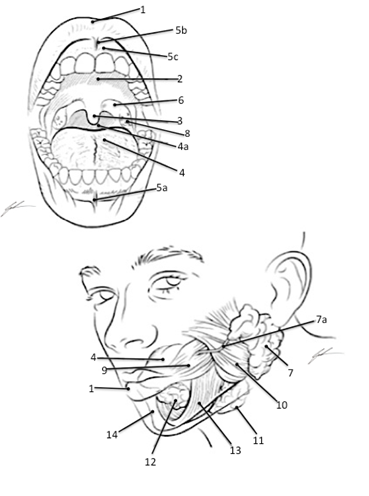
Tongue, Incisor, Canine (Cuspid) Pre-Molar (Bicuspid) Molar, Parotid Salivary Gland, Masseter, Temporalis
|
Structures of the Oral Opening Associated with Digestion 1 Labia (Lips) 2 Hard Palate 3 Uvula 4 Tongue 4a Esophagus 5a Inferior Labial Frenulum 5b Superior Labial Frenulum 5c Gingiva (Gums) 6 Palatopharyngeal Arch 7 Parotid Gland 7a Duct of Parotid Gland 8 Palatine Tonsils 9 Buccinator 10 Masseter 11 Submandibular Gland 12 Sublingual Gland 13 Mylohyoid 14 Mandible Color each structure, tissue or organ with a different color to assist with identification |
|
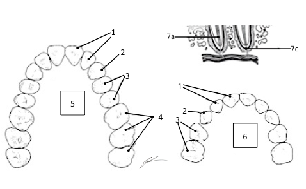
TEETH PATTERN (Omnivore Pattern of 2 Incisor:1Canine (Cuspid):2 Pre-Molar (Bicuspid):3 Molar per side of jaw and per bone of jaw) 1 Incisor 2 Canine (Cuspid) 3 Pre-Molar (Bicuspid) 4 Molar 5 Permanent (Adult) 6 Deciduous (Juvenile) Color each type of tooth a different color to assist with identification |
3. Select a “team leader” and use the colored images and reference materials to take turns labeling the sagittal head and mouth models
a. As structures are labeled, indicate the function of the structure or tooth being labeled.
|
Structure/Tooth |
Function |
|
Masseter Muscle |
|
|
Sublingual Gland |
|
|
Parotid Gland and Duct |
|
|
Submandibular Gland |
|
|
Tongue |
|
|
Incisor |
|
|
Canine (Cuspid) |
|
|
Pre-Molar (Bicuspid) |
|
|
Molar |
4. Have your instructor check your progress and clean-up your lab area.
ACTIVITY 3: Chemical Digestion of Foods
Purpose:
The following experiment will use various digestive enzymes and environmental conditions to examine the chemical digestion of foodstuff macromolecules (carbohydrates, lipids and proteins) into the smaller molecules that comprise the macromolecules that we consume within our food.
Background:
The function of the digestive system is to break down large food molecules (carbohydrates, proteins, lipids) into the smaller subunits that make them up. This process ensures molecules following digestion are small enough to be absorbed within the intestines and then transported through the vasculature of the body to the tissues and cells where those molecules can then be used. The means by which food is broken down following mastication and chemical digestion.
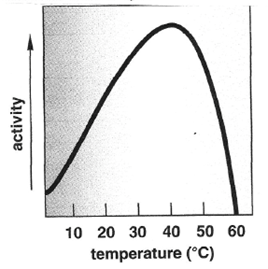
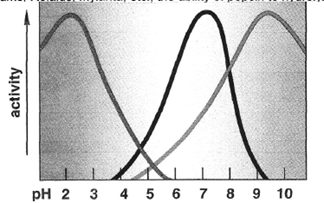
One must remember that enzymes are proteins and since enzymes are proteins, the environment that they are found will affect their effectiveness. For humans, digestive enzymes function the best at temperatures of 37oC and will be drastically impacted by temperatures above or below this temperature. Being heated above 60oC will lead to the protein being denatured and thus will no longer function properly. Conversely being cooled will progressively decrease activity for some enzymes to the point of no activity once a temperature of 0oC is reached. Additionally, enzymes will function within an optimal range based on pH sensitivity of the molecule. This pH sensitivity is an indication as to where within the pathway of digestion the enzyme will principally function. An example of this would-be pepsinogen that must be exposed to the acidic pH (2.0) within the gastric secretions to become active and digest proteins into short-polypeptide chains. Lastly, the ability for the enzymes within the polar environment of the alimentary canal to interact with non-polar substances will be dependent upon the emulsification that comes about through the exposure and interaction with bile salts.
|
Figure 1. The impact that changes in temperature and pH has on the activity of the digestive enzymes.
|
The Importance of Enzymes in Chemical Digestion
There are many different substances that are secreted into the different segments of the alimentary canal. Many of these substances facilitate the breakdown of food (i.e. mucus, bile salts, bilirubin, hydrochloric acid (HCl), and sodium bicarbonate (NaHCO3)). However, the most important substances secreted for the purpose of digestion are the digestive enzymes. Digestive enzymes greatly enhance the rate at which the covalent bonds that link subunits together to form polymeric biomolecules are broken. Indeed, without the presence of these enzymes, chemical digestion would essentially not occur. Although substances such as HCl and NaHCO3 can alter non-covalent bonding patterns within and among biomolecules, they typically cannot break down covalent bonds.
These enzymes within the alimentary canal (stomach and intestines) and secreted by the accessory organs (liver, pancreas, salivary glands, gall bladder) will perform the action of digestion based on specificity to a substrate. Where some enzymes will digest complex carbohydrates (polysaccharides) into simpler carbohydrates (disaccharides and monosaccharides), while others will only digest lipids (large fatty acids and triglycerides) into glycerol and free-fatty acids, and others will digest proteins into their amino acid units. In which an enzyme that digest the carbohydrate will not digest the lipid or the protein, and the enzyme for the lipid will not digest the carbohydrate or protein, nor will the enzyme for protein digestion digest the carbohydrate or lipid.
Carbohydrates are the first type of biomolecule to be chemically digested in the alimentary canal, as chemical digestion begins in the oral cavity through a salivary enzyme called salivary amylase (or ptyalin). Salivary amylase begins the breakdown of the polysaccharide amylose (starch, the principal storage carbohydrate in plants) into the disaccharide maltose. However, when food is swallowed and transferred to the small intestine, chemical digestion of carbohydrates effectively stops. Only when the chyme passes into the small intestine will carbohydrate digestion resume. In the small intestine the chyme is exposed to pancreatic amylase (which continues the process of breaking down starch and glycogen into disaccharides and oligosaccharides) and certain brush-border enzymes (e.g., lactase, sucrase, and maltase) that break down specific oligosaccharides into the monosaccharides that are absorbed by the intestinal epithelium.
Chemical digestion of protein begins in the stomach. The stomach produces a mixture of fluids called gastric juice in response to neural stimulation (induced by smell, site and taste of food), by distension of the stomach as food enters, and by pH changes induced as the more neutral pH food enters the acidic stomach. Gastric juice contains a number of substances, but the two most important for initiating protein digestion are hydrochloric acid (HCl) and the protease pepsin. HCl is secreted by parietal cells in the gastric mucosa. The low pH (~2) of the gastric juice aids protein digestion in a couple of ways. First, the low pH denatures the tertiary structures of ingested protein, making them easier to digest enzymatically. Secondly, the low pH is required for the activation of pepsin. Pepsin is produced by chief cells of the gastric pit in the molecule’s inactive form called pepsinogen, but once it diffuses into the lumen of the stomach the acidic conditions enable it to have a weak proteolytic activity). Pepsinogen can digest some ingested protein, but more importantly pepsinogen molecules will partially digest one another, removing inhibitory segments of the polypeptide chain and thus converting each other into the fully active enzyme pepsin. Pepsin breaks peptide bonds between amino acids with hydrophobic side chains in the middle of polypeptides, thus it cleaves long polypeptides into shorter polypeptides.
Although the chemical digestion of protein begins in the stomach through the actions of pepsin, most of the digestion of protein takes place in the small intestine. Indeed, individuals with complete gastrostomies can still completely digest protein, although the homogenization of chyme through chemical and physical digestion in the stomach aids this process. The proteases that function in the small intestine comes from two major sources: membrane bound enzymes on the brush-border of the intestinal mucosa, and enzymes secreted into the small intestine from the pancreas. The pancreatic enzymes, along with bicarbonate salts, are components of pancreatic juice that is secreted primarily when food enters the small intestine through the pyloric sphincter. Chemicals in the chyme induce cells in the small intestine to secrete the hormones secretin, which stimulates water and bicarbonate secretion in the pancreas, and cholecystokinin (CCK), which stimulates enzyme secretion in the pancreas. These hormones, in turn, cause the pancreas to release pancreatic juice through the duodenal papilla. A number of different proteases are found within pancreatic juice, but most are released as zymogens (inactive enzymes). Enzymes in the brush-border activate these zymogens, which ultimately digest the polypeptides into a combination of free amino acids, dipeptides, and oligopeptide that are absorbed by intestinal epithelium.
Lipids are a particularly challenging group of molecules to chemically digest, as fats being hydrophobic tend to aggregate into large droplets within the water environment of the alimentary canal. The result of the aggregation minimizes the surface area of contact between the fat and the surrounding water. Since the digestive enzymes are water soluble, they can only come into contact with and digest those triglyceride molecules at the surface of the droplets. Thus, for fat digestion to be efficient, the large droplets must be broken into much smaller droplets and held in these smaller droplets in order to increase surface area and enable enzymes (lipases) adequate contact with their substrate. Although there is a gastric lipase secreted in the stomach that causes a small amount of fat digestion, and infants produce a salivary lipase, almost all of the digestion of fat takes place in the small intestine. Like protein digestion, fat digestion is achieved through the activity of both pancreatic and intestinal brush border lipases. However, efficient fat digestion also involves the secretion of bile from the liver and gall bladder. Bile contains a mixture of bilirubin, cholesterol, phospholipids, inorganic ions, phospholipids, and negatively charged cholesterol derivatives called bile salts that functions to emulsify the lipids in the water of the alimentary canal assisting the digestion of the large fat droplets. The interaction with bile in the small intestine greatly increases the surface area over which lipases can come into contact with the triglycerides and hydrolyze them into amphipathic free fatty acids and monoglycerides, both of which can be absorbed by the intestinal epithelium. The liberation of free fatty acids, which are acids, would lead to a decrease in the pH of the fluid passing through the intestine, although normally the presence of bicarbonate in the pancreatic juice’s buffers against a decrease in pH.
Hypothesis: Generate a hypothesis regarding conditions that would best allow for digestion to occur.
Materials and Methods:
|
Test-tubes Wax Pencils Test-tube Rack pH paper |
Reagents: Distilled H2O 5% Starch Solution 5% Maltose /Dextrose Solution Egg White (Boiled) |
Digestive Enzymes & Regulators: Saliva (or 5% Amylase) 5% Pepsin 0.2 M HCl 0.2 M NaHCO3 |
Indictors: IKI-solution Benedict’s Solution |
Methods:
Complete the digestion activity as assigned by your instructor. Following completion of the digestion you will share results with the other members in your group.
Activity 1: Assessing Starch Digestion by Salivary Amylase.
1. From the general supply area, pipette distilled water, maltose, amylase and starch solutions as indicated into the appropriate test tube as indicated in Table 1A
2. Once all tubes have been made, add the appropriate HCl or NaHCO3 solutions into test tubes 6A-11A as indicated in Table 1A
Table 1A. Set-up for each test-tube for digestion of starch by amylase.
|
Tube |
Reagent and Amount |
Reagent and Amount |
|
1A |
5 mL- Starch Solution |
|
|
2A |
5mL-Distilled Water |
|
|
3A |
5 mL- Maltose/Dextrose Solution |
|
|
4A |
5 mL- Starch Solution |
|
|
5A |
5 mL- Starch Solution |
|
|
6A |
5 mL- Starch Solution |
5 mL- HCl |
|
7A |
5 mL- Starch Solution |
5 mL- HCl |
|
8A |
5 mL- Starch Solution |
5 mL- NaHCO3 |
|
9A |
5 mL- Starch Solution |
5 mL- NaHCO3 |
|
10A |
5 mL- Maltose/Dextrose Solution |
5 mL- HCL |
|
11A |
5 mL- Maltose/Dextrose Solution |
5 mL- NaHCO3 |
3. From your supplies, obtain your pH indicator paper. Get 1 strip for each test tube and place 1 drop of each solution on the test strip. Record pH of solutions in Table 3.
4. Using a clean transfer pipette, transfer 5-mL of the collect saliva into test tubes 4A, 5A, 6A, 7A, 8A, and 9A (Make sure not to touch the solutions with the pipette that you are using to transfer the saliva)
5. Cap the test tubes and shake to mix for 30-seconds
6. Set timer for 30-minutes
7. Transfer test tube into test tube rack in incubation temperature as indicated in Table 1B. Make sure to submerge the test tube in the incubation bath, for ice this may mean making a small hole in the ice with your finger. Once all test tubes are submerged start your timer.
Table 1B- Incubation temperature for chemical digestion of carbohydrates by salivary amylase.
|
Test Tube |
1A |
2A |
3A |
4A |
5A |
6A |
7A |
8A |
9A |
10A |
11A |
|
Incubation Condition |
Warm Water Bath |
Warm Water Bath |
Warm Water Bath |
Warm Water Bath |
Ice Water Bath |
Warm Water Bath |
Ice Water Bath |
Warm Water Bath |
Ice Water Bath |
Warm Water Bath |
Warm Water Bath |
|
Temperature (oC) |
37oC |
37oC |
37oC |
37oC |
0oC |
37oC |
0oC |
37oC |
0oC |
37oC |
37oC |
8. While the solutions are incubating, from the general supply area, obtain a test tube rack, 11 test tubes, IKI, Benedict’s solution
9. Label tests as indicated 1A, 2A, 3A, 4A, 5A, 6A, 7A, 8A, 9A, 10A, 11A
10. After 30-minutes, remove the tubes from the water bath or ice bath
11. Divide the contents of each tube into two using the second test tube that you just labeled.
12. For each pair of test tubes:
a. Take the original test tube and add 2-to-3 drops of IKI
i. Agitate the test tube to mix the solution with the IKI and record the color in Table 3.
b. In the second test tube add 5-drops of Benedict’s solution.
i. Place test tube in the boiling water bath for 5-mintues (should see color change within the solution)
ii. Carefully using the test tube holders remove the test tubes and return to your work area, record your results in Table 3.
As a group, think about: Why do you need to test dextrose (maltose) solution in IKI and Benedict’s Solution? Share your thoughts with your instructor
Activity 2: Assessing Protein Digestion by Pepsin.
1. From the general supply area obtain 8 clean test tubes, and pipettes
2. Label test tubes 1P, 2P, 3P, 4P, 5P, 6P, 7P, 8P
3. Cut the cooked egg white into small slices (approximately 1 mm thick)
4. Using a toothpick, careful transfer 5 slices and place into test tube 1P, tamp down into the test tube so that the egg is suspended in the solution.
5. Repeat for each test tube until all 8 have egg (Make sure that you are using a different toothpick for each test tube)
6. Fill test tubes as indicated in Table 2A
7. Using a different transfer pipette for each solution or reagent transfer the correct amount into each tub based on the following table for amounts of solutions and reagents to place in each test tube. Make sure to shake to ensure that the solution is homogenous. When drawing solution, begin from the bottom and continuously draw into the pipette as you pull the pipette out of the bottle.
Table 2A. Set-up for each test-tube to perform the digestion of proteins by pepsin.
|
Tube |
Reagent and Amount |
Reagent and Amount |
|
1P |
10 mL- DiH2O |
|
|
2P |
5 mL- Pepsin |
|
|
3P |
5 mL- Pepsin |
5 mL- HCl |
|
4P |
5 mL- Pepsin |
5 mL- NaHCO3 |
|
5P |
10 mL- DiH2O |
|
|
6P |
5 mL- Pepsin |
|
|
7P |
5 mL- Pepsin |
5 mL- HCl |
|
8P |
5 mL- Pepsin |
5 mL- NaHCO3 |
8. When dispensing Pepsin into test tubes 2P, 3P, 4P, 6P, 7P, 8P.
a. Make sure to shake the bottle with Pepsin to ensure solution is homogenous
b. When drawing, begin to draw from the bottom and continue to draw in Pepsin as you slowly pull pipette from bottle.
c. When dispensing into the test-tube, be careful to not allow the pipette to touch the solution in the test tube.
9. From your supplies, obtain your pH indicator paper. Get 1 strip for each test tube and place 1 drop of each solution on the test strip. Record pH of solutions in Table 4.
10. Cap the test tube and shake for 30-seconds to mix the solution
11. Set timer for 15-minutes.
12. Transfer test tube into test tube rack in incubation temperature as indicated in Table 1B. Make sure to submerge the test tube in the incubation bath, for ice this may mean making a small hole in the ice with your finger. Once all test tubes are submerged start your timer.
Table 2B- Incubation temperature for chemical digestion of carbohydrates by salivary amylase.
|
Test Tube |
1P |
2P |
3P |
4P |
5P |
6P |
7P |
8P |
|
Incubation Condition |
Warm Water |
Warm Water |
Warm Water |
Warm Water |
Ice Water |
Ice Water |
Ice Water |
Ice Water |
|
Temperature (oC) |
37oC |
37oC |
37oC |
37oC |
0oC |
0oC |
0oC |
0oC |
13. After 15-minutes, remove the tubes from the water bath or ice bath and examine the eggs for indication of digestion. Record your observations in table 4.
14. Return to the correct incubation.
15. Repeat the timer for 15-minutes examining the egg at 15-minute intervals for signs of digestion for a total of 1-hour.
16. At the end of the incubation period, remove test tube for the ice or warm water
17. Do a final examination of the eggs for signs of digestion. Record your results in the table 4.
As a group, think about: Why do you need to test a control solution in the experiment? What test tube is the control solution in this activity? Share your thoughts with your instructor
Results
Table 3 Results of the digestion of starch by amylase based on testing condition.
|
Test Tube |
pH of Solution |
IKI Test (Color) |
Indication of Digestion of Starch (yes/no) |
Kind of carbohydrate-solution do you have after 30-minutes of digestion |
|
1A |
||||
|
2A |
||||
|
3A |
||||
|
4A |
||||
|
5A |
||||
|
6A |
||||
|
7A |
||||
|
8A |
||||
|
9A |
||||
|
10A |
||||
|
11A |
*Note that the digestive enzyme solution has glucose present within the solution. TEST COLOR INDICATOR: IKI: Purple=Starch, Red=Dextrin, Tan/Yellow=Maltose; Benedict’s Solution: Orange/Yellow = disaccharide present (low, moderate, high), Green= trace amounts of disaccharide, Blue= no disaccharide (negative)
Table 4. Results of protein digestion based on different conditions.
|
Test Results |
1P |
2P |
3P |
4P |
5P |
6P |
7P |
8P |
|
pH of solution |
||||||||
|
Initial Observation (Time: 0-min) |
||||||||
|
Observation (Time: 15-min) |
||||||||
|
Observation (Time: 30-min) |
||||||||
|
Observation (Time: 45-min) |
||||||||
|
Observation (Time: 60-min) |
||||||||
|
Determination of digestion of egg protein at the end of 60-minutes (Yes/No) |
If digestion has occurred, egg white will begin to fray and dissolve within the test tube. You will notice fraying at the edges and “bubbles” within the solid egg white, there may also be “wisps” of white material (looks like clouds) with the solution in the test tube. To evaluate digestion, you may need to agitate test tube by gently shaking side-to-side for a few seconds.
Discussion: In a coherent paragraph discuss your results (Think about: What can you conclude about the impact that environmental conditions had on the enzymes, relative to their functionality? How will the results for digestion by the various enzymes allow you to determine the area of activity within anatomy of the gastrointestinal system for each enzyme? In your discussion, address how temperature of the reaction, addition of co-factors of the enzyme each affected the direction of the reaction and what this means about where activity of the enzymes might be highest/lowest within the totality of the alimentary canal.)