11: Microbial Nutrition
- Page ID
- 10681
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)All microbes have a need for three things: carbon, energy, and electrons. There are specific terms associated with the source of each of these items, to help define organisms.
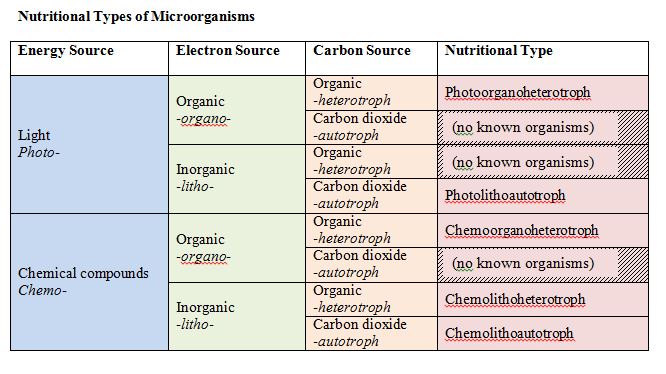
Let us focus on carbon first. All organisms are carbon-based with macromolecules – proteins, carbohydrates, lipids, nucleic acid – having a fundamental core of carbon. On one hand, organisms can use reduced, preformed organic substances as a carbon source. These are the heterotrophs or “other eaters.” Alternatively, they can rely on carbon dioxide (CO2) as a carbon source, reducing or “fixing” it this inorganic form of carbon into an organic molecule. These are the autotrophs or “self feeders.”
For energy, there are two possibilities as well: light energy or chemical energy. Light energy comes from the sun, while chemical energy can come from either organic or inorganic chemicals. Those organisms that use light energy are called phototrophs (“light eaters”), while those that use chemical energy are called chemotrophs (“chemical eaters”). Chemical energy can come from inorganic sources or organic sources. An organism that uses inorganic sources is known as a lithotroph (“rock eater”), while an organism that uses organic sources is called an organotroph (“organic eater”).
These terms can all be combined, to derive a single term that gives you an idea of what an organism is using to meet its basic needs for energy, electrons, and carbon.
Macronutrients
In addition to carbon, hydrogen and oxygen, cells need a few other elements in sufficient quantity. In particular, cells need nitrogen for the formation of proteins, nucleic acids, and a few other cell components. Cells also need phosphorous, which is a crucial component of nucleic acids (think sugar-phosphate backbone!), phospholipids, and adenosine triphosphate or ATP. Sulfur is necessary for a few amino acids, as well as several vitamins, while potassium is needed for enzymes, and magnesium is used to stabilize ribosomes and membrane. Collectively these elements (including C, H, and O) are referred to as the macronutrients.
Growth Factors
Some microbes can synthesize certain organic molecules that they need from scratch, as long as they are provided with carbon source and inorganic salts. Other microbes require that certain organic compounds exist within their environment. These organic molecules essential for growth are called growth factors and fall in three categories: 1) amino acids (building blocks of protein), 2) purines and pyrimidines (building blocks of nucleic acid), and 3) vitamins (enzyme cofactors).
Uptake of Nutrients
In order to support its’ activities, a cell must bring in nutrients from the external environment across the cell membrane. In bacteria and archaea, several different transport mechanisms exist.
Passive Diffusion
Passive or simple diffusion allows for the passage across the cell membrane of simple molecules and gases, such as CO2, O2, and H2O. In this case, a concentration gradient must exist, where there is higher concentration of the substance outside of the cell than there is inside the cell. As more of the substance is transported into the cell the concentration gradient decreases, slowing the rate of diffusion.
Facilitated Diffusion
Facilitated diffusion also involves the use of a concentration gradient, where the concentration of the substance is higher outside the cell, but differs with the use of carrier proteins (sometimes called permeases). These proteins are embedded within the cell membrane and provide a channel or pore across the membrane barrier, allowing for the passage of larger molecules. If the concentration gradient dissipates, the passage of molecules into the cell stops. Each carrier protein typically exhibits specificity, only transporting in a particular type of molecule or closely related molecules.
Active Transport
Many types of nutrient uptake require that a cell be able to transport substances against a concentration gradient (i.e. with a higher concentration inside the cell than outside). In order to do this, a cell must utilize metabolic energy for the transport of the substance through carrier proteins embedded in the membrane. This is known as active transport. All types of active transport utilize carrier proteins.

Active Transport Versus Facilitated Diffusion.
Primary active transport
Primary active transport involves the use of chemical energy, such as ATP, to drive the transport. One example is the ABC system, which utilizes ATP-Binding Cassette transporters. Each ABC transporter is composed of three different components: 1) membrane-spanning proteins that form a pore across the cell membrane (i.e. carrier protein), 2) an ATP binding region that hydrolyzes ATP, providing the energy for the passage across the membrane, and 3) a substrate-binding protein, a peripheral protein that binds to the appropriate substance to be transporter and ferries it to the membrane-spanning proteins. In gram negative bacteria the substrate-binding protein is located in the cell’s periplasm, while in gram positive bacteria the substrate-binding protein is attached to the outside of the cell membrane.

ABC Transporter Structure.
Secondary active transport
Secondary active transport utilizes energy from a proton motive force (PMF). A PMF is an ion gradient that develops when the cell transports electrons during energy-conserving processes. Positively charged protons accumulate along the outside of the negatively charged cell, creating a proton gradient between the outside of the cell and the inside.
There are three different types of transport events for simple transport: uniport, symport, and antiport and each mechanism utilizes a different protein porter. Uniporters transport a single substance across the membrane, either in or out. Symporters transport two substances across the membrane at the same time, typically a proton paired with another molecule. Antiporters transport two substances across the membrane as well, but in opposite directions. As one substance enters the cell, the other substance is transported out.

Uniport Synport Antiport. By Lupask (Own work) [Public domain], via Wikimedia Commons
Group Translocation
Group translocation is a distinct type of active transport, using energy from an energy-rich organic compound that is not ATP. Group translocation also differs from both simple transport and ABC transporters in that the substance being transported is chemically modified in the process.
One of the best studied examples of group translocation is the phosphoenolpyruvate: sugar phosphotransferase system (PTS), which uses energy from the high-energy molecule phosphoenolpyruvate (PEP) to transport sugars into the cell. A phosphate is transferred from the PEP to the incoming sugar during the process of transportation.

Group Translocation via PTS.
Iron Uptake
Iron is required by microbes for the function of their cytochromes and enzymes, resulting in it being a growth-limiting micronutrient. However, little free iron is available in environments, due to its insolubility. Many bacteria have evolved siderophores, organic molecules that chelate or bind ferric iron with high affinity. Siderophores are released by the organism to the surrounding environment, whereby they bind any available ferric iron. The iron-siderophore complex is then bound by a specific receptor on the outside of the cell, allowing the iron to be transported into the cell.

Siderophores and Receptor Sites.
Key Words
heterotroph, autotroph, phototroph, chemotroph, lithotroph, organotroph, photolithoautotroph, photoorganoheterotroph, chemoorganoheterotroph, chemolithoautotroph, chemolithoheterotroph, macronutrients, growth factors, passive/simple diffusion, facilitated diffusion, carrier protein/permease, active transport, primary active transport, ABC system, ATP-binding cassette transporter, ABC transporter, secondary active transport, proton motive force (PMF), uniport, symport, antiport, porter, uniporter, symporter, antiporter, group translocation, phosphoenolpyruvate: sugar phosphotransferase system (PTS), phosphoenolpyruvate (PEP), siderophore.
Study Questions
- What are the different terms associated with microbial nutritional types? How can these terms be combined to define the nutritional types of microbes in terms of their sources of carbon, electrons, and energy?
- What are macroelements and why are they important to a cell? What are growth factors and what is their significance to a cell?
- What is the importance of nutrient uptake for a cell? What are the common features of nutrient uptake by bacteria?
- What is transported into a bacteria cell by passive diffusion and how does this affect a bacterial cell?
- Explain diffusion (passive and facilitated) and active transport.
- What are the 3 types of active transport? Be able to diagram each processes. What is required for each of these processes? How are they similar, how are they different?
- Why is iron uptake important for a cell? What is used to accomplish this?
Exploratory Questions (OPTIONAL)
- What is the largest bacterium or archaean ever discovered? What is the smallest eukaryote ever discovered?


