4.1.2: Aerobic Cellular Respiration
- Page ID
- 50320
Learning Objectives
- Identify the reactants and products of aerobic cellular respiration.
- Explain each step of aerobic cellular respiration and where in the cell it occurs.
Not only do plants produce sugars through photosynthesis, but they also break down these sugars to generate usable energy in the form of ATP through aerobic cellular respiration. Glucose begins its breakdown outside of the mitochondria in a metabolic pathway called glycolysis. However, the majority of the reactions that produce ATP happen within the mitochondria (in eukaryotic cells; Figure \(\PageIndex{1}\)). During these reactions, electron carriers are created and oxygen pulls the electrons through an electron transport chain to create ATP, which powers cellular activity. The oxygen you breathe in combines with electrons to form water, which you breathe out. The carbon dioxide you breathe out comes from the carbon in glucose, which your body metabolized.
Here is a net reaction for cellular respiration:
\[\ce{C_6H_{12}O_6 + 6O_2\rightarrow6CO_2 + 6H_2O + ATP} \nonumber\]
glucose + oxygen \(\ce{\rightarrow}\) carbon dioxide + water + energy
Step 1: Glycolysis
When glucose is transported into the cytoplasm of cells, it is broken down into two molecules of pyruvate (Figure \(\PageIndex{2}\)). This process is called glycolysis (glyco- for glucose and -lysis, meaning to break apart). Glycolysis involves the coordinated action of many different enzymes. As these enzymes start to break the glucose molecule apart, an initial input of energy is required. This initial energy is donated by molecules of ATP.
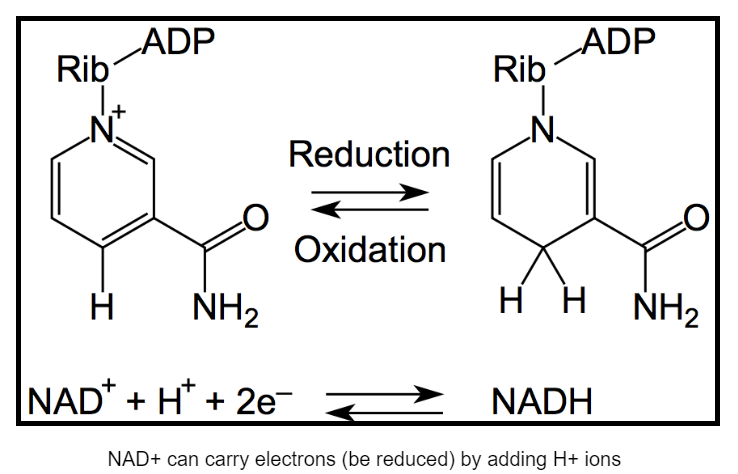
Though two molecules of ATP are used to get glycolysis going, four more molecules of ATP are produced during the reaction, resulting in the net production of two ATP per molecule of glucose. In addition to ATP, two molecules of nicotinamide adenine dinucleotide (NAD+) are reduced to form NADH (Figure \(\PageIndex{3}\)). When NAD+ is reduced to NADH, two high energy electrons derived from breaking the bonds of glucose are added to it. One of those negatively charged electrons is balanced by the positive charge (+) on NAD+. The other is balanced by adding a proton (H+) to the molecule.
Step 2: Pyruvate oxidation
If oxygen is present, aerobic cellular respiration can continue. The two molecules of pyruvate are transported into the matrix of the mitochondrion. During transport, each pyruvate is converted into a 2-carbon molecule called acetyl-\(\ce{CoA}\). The other carbon atom from each pyruvate molecule exits the cell as \(\ce{CO2}\). The electrons from this broken bond are captured by another molecule of NAD+, reducing it to NADH. Because two molecules of pyruvate are produced from each glucose molecule during glycolysis, two acetyl CoA molecules are produced (one from each pyruvate) during pyruvate oxidation (Figure \(\PageIndex{4}\)).
Step 3: The Citric Acid (Krebs) Cycle
The two acetyl-\(\ce{CoA}\) molecules enter a cycle which, much like glycolysis, involves the action of many different enzymes to release energy and transport it in energy-carrying molecules, including 2 ATP, 6 NADH, and 2 \(\ce{FADH2}\), another electron carrier (Figure \(\PageIndex{4}\)). This cycle takes place within the matrix of the mitochondrion.
Step 4: Oxidative Phosphorylation
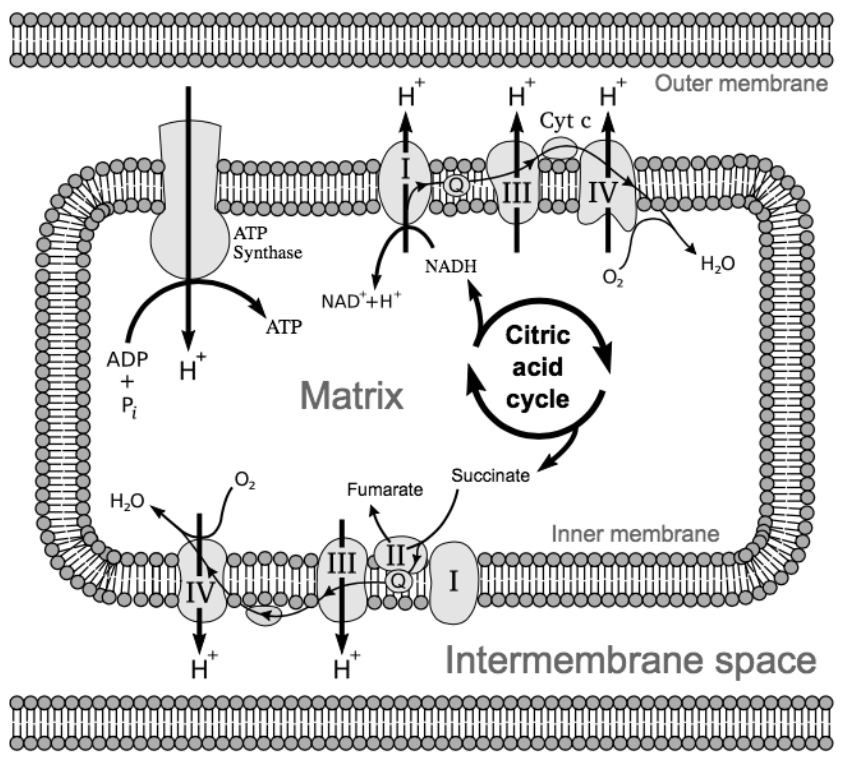
This stage of cellular respiration has two steps. During the electron transport chain, our electron carriers power a series of proton pumps that move \(\ce{H+}\) ions from the mitochondrial matrix to the space between the inner and outer mitochondrial membranes. During chemiosmosis, an enzyme called ATP synthase allows the protons to flow back into the mitochondrial matrix, using the physical flow of the protons to turn ADP into ATP.
The Electron Transport Chain
NADH and \(\ce{FADH2}\) drop off their electrons at a protein complex within the inner mitochondrial membrane. This effectively “turns on” this protein complex, which pumps a \(\ce{H+}\) from the mitochondrial matrix to the intermembrane space. The electrons are then passed down a line of protein complexes, much like a current of electricity, powering these complexes to each pump a \(\ce{H+}\) from the matrix into the intermembrane space. This is appropriately named the electron transport chain (Figure \(\PageIndex{5}\)).
At the end of the electron transport chain, the low energy electrons need to be picked up to make space for more electrons. An oxygen atom picks up two electrons and, to balance the charge, two \(\ce{H+}\) from the matrix, forming a water molecule (\(\ce{H2O}\)). In cellular respiration, oxygen is the terminal electron acceptor, because it picks up the electrons at the end (the terminus) of the electron transport chain. This job is so important that, as you saw above, if oxygen is not present, this part of cellular respiration will not occur.
Chemiosmosis
Why are the protein complexes pumping \(\ce{H+}\) into the intermembrane space? The intermembrane space is relatively small. As more \(\ce{H+}\) are added to this area, the intermembrane space becomes increasingly positively charged, while the matrix becomes increasingly negatively charged. This is similar to how a battery stores energy--by creating an electrochemical gradient. The positive charges repel each other and would “prefer” to be balanced across both sides of the membrane. However, they cannot directly pass through the membrane. Even though they are small, \(\ce{H+}\) ions carry a full charge, making them too polar to pass through the nonpolar tails of the phospholipid bilayer that composes the mitochondrial membranes.
An enzyme called ATP synthase allows the \(\ce{H+}\) to move back into the matrix. This enzyme is structured much like a waterwheel or turbine -- the flow of protons through the enzyme physically rotates it, converting the potential energy stored in the electrochemical gradient into kinetic energy (movement)! This kinetic energy is used to force another phosphate group onto ADP, converting the kinetic energy back into chemical energy, which is stored in the bonds of ATP
Attributions
Curated and authored by Melissa Ha using the following sources:
- Kammy Algiers (CC-BY-NC)
- 13.3: Cellular Respiration in Botany Lab Manual by Maria Morrow (CC-BY)

