C4: Lipid Conformational Transitions
- Page ID
- 4681
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\( \newcommand{\dsum}{\displaystyle\sum\limits} \)
\( \newcommand{\dint}{\displaystyle\int\limits} \)
\( \newcommand{\dlim}{\displaystyle\lim\limits} \)
\( \newcommand{\id}{\mathrm{id}}\) \( \newcommand{\Span}{\mathrm{span}}\)
( \newcommand{\kernel}{\mathrm{null}\,}\) \( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\) \( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\) \( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\id}{\mathrm{id}}\)
\( \newcommand{\Span}{\mathrm{span}}\)
\( \newcommand{\kernel}{\mathrm{null}\,}\)
\( \newcommand{\range}{\mathrm{range}\,}\)
\( \newcommand{\RealPart}{\mathrm{Re}}\)
\( \newcommand{\ImaginaryPart}{\mathrm{Im}}\)
\( \newcommand{\Argument}{\mathrm{Arg}}\)
\( \newcommand{\norm}[1]{\| #1 \|}\)
\( \newcommand{\inner}[2]{\langle #1, #2 \rangle}\)
\( \newcommand{\Span}{\mathrm{span}}\) \( \newcommand{\AA}{\unicode[.8,0]{x212B}}\)
\( \newcommand{\vectorA}[1]{\vec{#1}} % arrow\)
\( \newcommand{\vectorAt}[1]{\vec{\text{#1}}} % arrow\)
\( \newcommand{\vectorB}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\( \newcommand{\vectorC}[1]{\textbf{#1}} \)
\( \newcommand{\vectorD}[1]{\overrightarrow{#1}} \)
\( \newcommand{\vectorDt}[1]{\overrightarrow{\text{#1}}} \)
\( \newcommand{\vectE}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash{\mathbf {#1}}}} \)
\( \newcommand{\vecs}[1]{\overset { \scriptstyle \rightharpoonup} {\mathbf{#1}} } \)
\(\newcommand{\longvect}{\overrightarrow}\)
\( \newcommand{\vecd}[1]{\overset{-\!-\!\rightharpoonup}{\vphantom{a}\smash {#1}}} \)
\(\newcommand{\avec}{\mathbf a}\) \(\newcommand{\bvec}{\mathbf b}\) \(\newcommand{\cvec}{\mathbf c}\) \(\newcommand{\dvec}{\mathbf d}\) \(\newcommand{\dtil}{\widetilde{\mathbf d}}\) \(\newcommand{\evec}{\mathbf e}\) \(\newcommand{\fvec}{\mathbf f}\) \(\newcommand{\nvec}{\mathbf n}\) \(\newcommand{\pvec}{\mathbf p}\) \(\newcommand{\qvec}{\mathbf q}\) \(\newcommand{\svec}{\mathbf s}\) \(\newcommand{\tvec}{\mathbf t}\) \(\newcommand{\uvec}{\mathbf u}\) \(\newcommand{\vvec}{\mathbf v}\) \(\newcommand{\wvec}{\mathbf w}\) \(\newcommand{\xvec}{\mathbf x}\) \(\newcommand{\yvec}{\mathbf y}\) \(\newcommand{\zvec}{\mathbf z}\) \(\newcommand{\rvec}{\mathbf r}\) \(\newcommand{\mvec}{\mathbf m}\) \(\newcommand{\zerovec}{\mathbf 0}\) \(\newcommand{\onevec}{\mathbf 1}\) \(\newcommand{\real}{\mathbb R}\) \(\newcommand{\twovec}[2]{\left[\begin{array}{r}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\ctwovec}[2]{\left[\begin{array}{c}#1 \\ #2 \end{array}\right]}\) \(\newcommand{\threevec}[3]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\cthreevec}[3]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \end{array}\right]}\) \(\newcommand{\fourvec}[4]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\cfourvec}[4]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \end{array}\right]}\) \(\newcommand{\fivevec}[5]{\left[\begin{array}{r}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\cfivevec}[5]{\left[\begin{array}{c}#1 \\ #2 \\ #3 \\ #4 \\ #5 \\ \end{array}\right]}\) \(\newcommand{\mattwo}[4]{\left[\begin{array}{rr}#1 \amp #2 \\ #3 \amp #4 \\ \end{array}\right]}\) \(\newcommand{\laspan}[1]{\text{Span}\{#1\}}\) \(\newcommand{\bcal}{\cal B}\) \(\newcommand{\ccal}{\cal C}\) \(\newcommand{\scal}{\cal S}\) \(\newcommand{\wcal}{\cal W}\) \(\newcommand{\ecal}{\cal E}\) \(\newcommand{\coords}[2]{\left\{#1\right\}_{#2}}\) \(\newcommand{\gray}[1]{\color{gray}{#1}}\) \(\newcommand{\lgray}[1]{\color{lightgray}{#1}}\) \(\newcommand{\rank}{\operatorname{rank}}\) \(\newcommand{\row}{\text{Row}}\) \(\newcommand{\col}{\text{Col}}\) \(\renewcommand{\row}{\text{Row}}\) \(\newcommand{\nul}{\text{Nul}}\) \(\newcommand{\var}{\text{Var}}\) \(\newcommand{\corr}{\text{corr}}\) \(\newcommand{\len}[1]{\left|#1\right|}\) \(\newcommand{\bbar}{\overline{\bvec}}\) \(\newcommand{\bhat}{\widehat{\bvec}}\) \(\newcommand{\bperp}{\bvec^\perp}\) \(\newcommand{\xhat}{\widehat{\xvec}}\) \(\newcommand{\vhat}{\widehat{\vvec}}\) \(\newcommand{\uhat}{\widehat{\uvec}}\) \(\newcommand{\what}{\widehat{\wvec}}\) \(\newcommand{\Sighat}{\widehat{\Sigma}}\) \(\newcommand{\lt}{<}\) \(\newcommand{\gt}{>}\) \(\newcommand{\amp}{&}\) \(\definecolor{fillinmathshade}{gray}{0.9}\)If a liposome preparation is placed in a sensitive calorimeter and the temperature slowly increased, it is observed that the liposome preparation absorbs a significant amount of heat at a temperature characteristic of the PL which compose the liposome. This is analogous to what would happen if solid water was heated. At the melting point of water, an increment of heat is required, the heat of fusion, to break H-bonds and cause melting. Likewise the heat of vaporization is measured when H-bonds are broken on the liquid-gas transition. These transition are associated with non-covalent processes, namely, breaking H-bonds. Graphs of heat absorbed (Q) as a function of temperature, or heat absorbed/T (i.e. the heat capacity) vs temperature are shown below for the melting and evaporation of water, and for liposome transitions.
Figure: Heat absorption and water: phase transitions
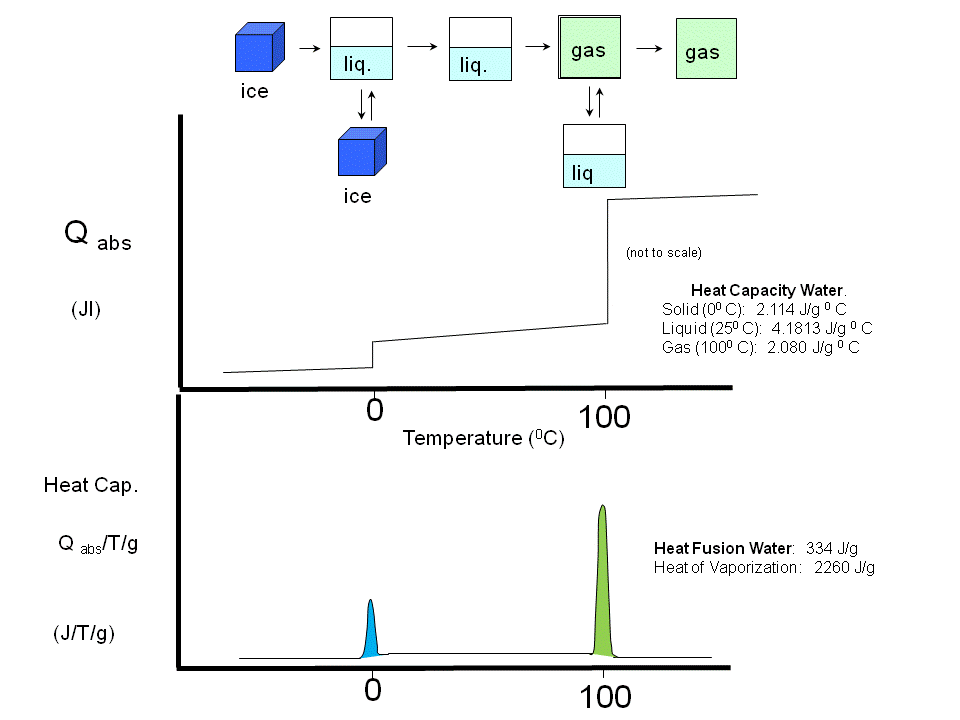
Figure: Differential Scanning Calorimeter (DSC): Phase transition for DPPC (Dipalmitoyl phosphatidylcholine)
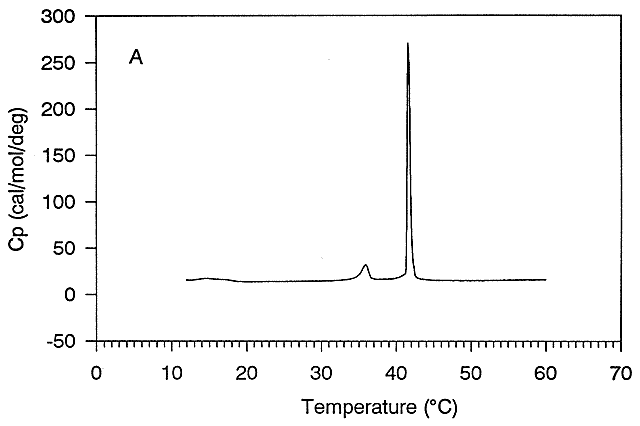
The transition observed with liposomes is caused by conformational chains in the packing of the acyl chains of the PLs, as the acyl chains change from trans to gauche conformations. These change involve not the simple translation of lipid molecules within and between bilayers but rather the movement of atoms within the molecules. These kinds of motions can be modeled using molecular dynamics simulations.
Before the transition, the acyl chains are more tightly packed in the gel phase, and after they are less tightly packed in the liquid crystalline phase, since many chains are in the gauche conformation.
Differences in Gel and Liquid Crystalline Phases in Phospholipids Bilayers
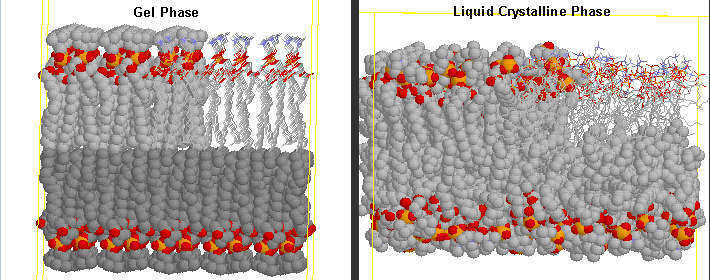
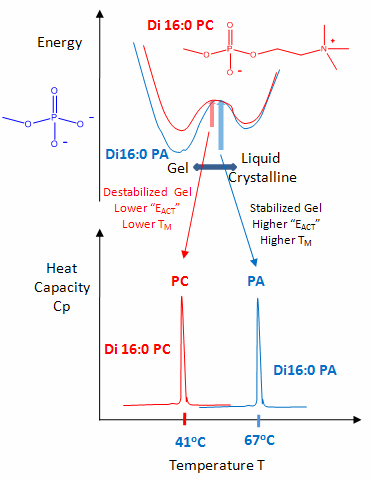
The midpoint of the phase transition is called the melting point, Tm. Vesicles in the liquid crystalline phase are more fluid, dynamic, and hence more permeable. Note that the liposomes have not been destroyed but simply have undergone a phase change, much like ice turning to liquid water. Liposomes made of different PL have different Tm.
Vesicles make from PLs with bigger head groups have a lower Tm, since they are less "stable". For example, the Tm for vesicles of di-16:0 versions of PA, PE, and PC have Tms of 67, 63, and 41 degrees C, respectively.
- TM di16:0 PC and di16:0 PA - Energy Diagram
Cholesterol is also a ubiquitous component of animal cell membranes. Its size will allow it to fit into either leaflet with its polar OH pointed to the outside. One function of cholesterol in membranes is to keep the membrane fluid at any reasonable temperature. When a membrane is at a temperature less than the Tm, it is ordinarily in a gel, not liquid crystalline phase. The cholesterol helps prevent ordered packing of the acyl chains of the PL's, which increases their freedom of motion. Hence the fluidity and permeability of the membrane is increased. At temperatures greater than the Tm, the rigid ring of cholesterol reduces the freedom of the acyl chains to rotation, and hence decreases the number of chains in the gauche conformation. This decreases the fluidity and permeability.
![]()
Both files from Scott Feller, Wabash College
Check out the following dynamics simulation of a bilayer membrane, which gives a clue into the way in which solutes could cross a membrane bilayer.
 Molecular Dynamics Simulation of a Hydrated DLPE Bilayer from DARMSTADT UNIVERSITY OF TECHNOLOGY, DEPARTMENT OF CHEMISTRY
Molecular Dynamics Simulation of a Hydrated DLPE Bilayer from DARMSTADT UNIVERSITY OF TECHNOLOGY, DEPARTMENT OF CHEMISTRY
Prof. Dr. J. Brickmann Group
Large Scale Conformational Transitions
Large scale conformational transitions can occur in lipid bilayers. These include bud formation, formation of vesicles which split off from the membrane, and fusion of membrane vesicles, all of which are important biologically. To some extent, the tendency to engage in these larger shape transitions depends on the local curvature of the vesicle, which depends on the local phospholipid composition and fluidity of the membrane. We have previously seen that segregation of lipids into rafts (or domains) within a leaflet depends on the phospholipid content. Baumgart et al. have made giant unilamellar vesicles (GUV) containing a mixture of three lipids: sphingomyelin (SM), dioleylphosphatidylcholine (DOPC), and cholesterol (C) to study transitions in the bilayers. SM and C segregate laterally into a "liquid phase" domain characterized by significant order (Lo) while DOPC forms a "liquid phase" with more disorder (Ld). They could detect the different phases through fluorescence microscopy of GUV's labeled with fluorophores (molecules that absorb visible or UV light and emit photons of lower energy (higher wavelength). The first, N-lissamine rhodamine dipalmitoylphosphatidylethanolamine (red fluorescence), partitions almost exclusively into the Ld phase. The second, perylene (blue fluorescence), partitioned with great selectivity into the Lo phase. The results of their studies are shown in the figures below. This images provide stunning visualization of lipid domains (rafts) including those in the shapes of circles, stripes, and rings. Their works shows that the boundary between the domains is important in determining lipid structures. Structures are favored which reduce the perimeter at the boundaries of the phases. It appears that the Ld phase (and associated lipids) is found preferentially in area of high membrane curvature, while the Lo phase is found is regions of lower curvature. The two figures below are reprinted with permission of Nature and the authors: Baumgart, Hess, and Webb, W. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature. 425, pg 821 (2003)
Figure: Equatorial cross sections of GUVs with multiple phases
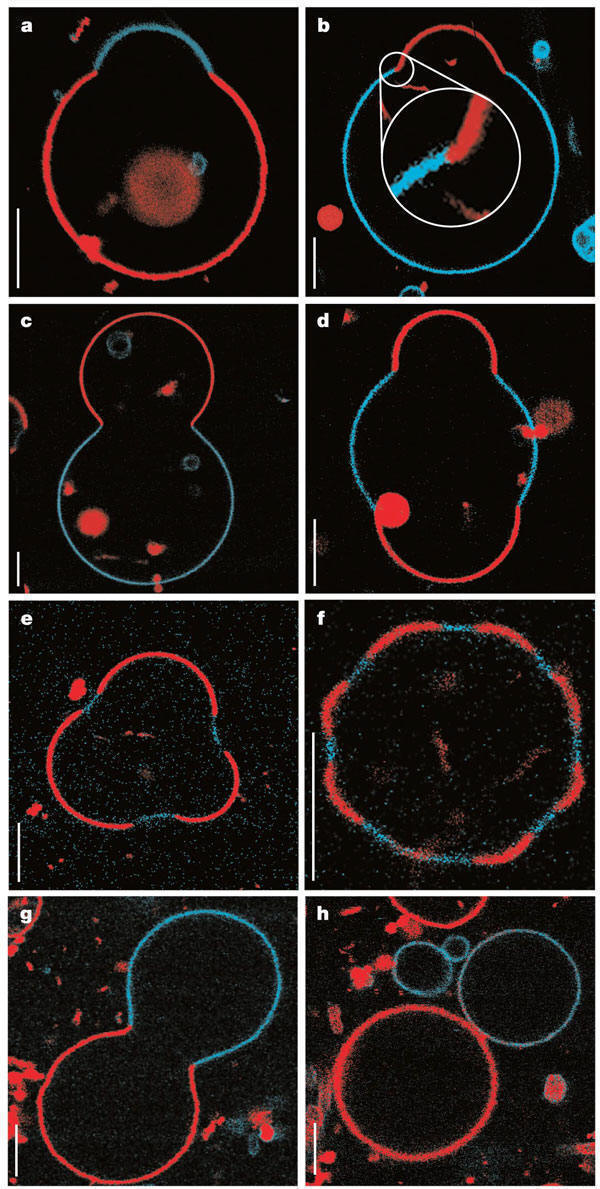
Figure: GUVs structures with multiple phases
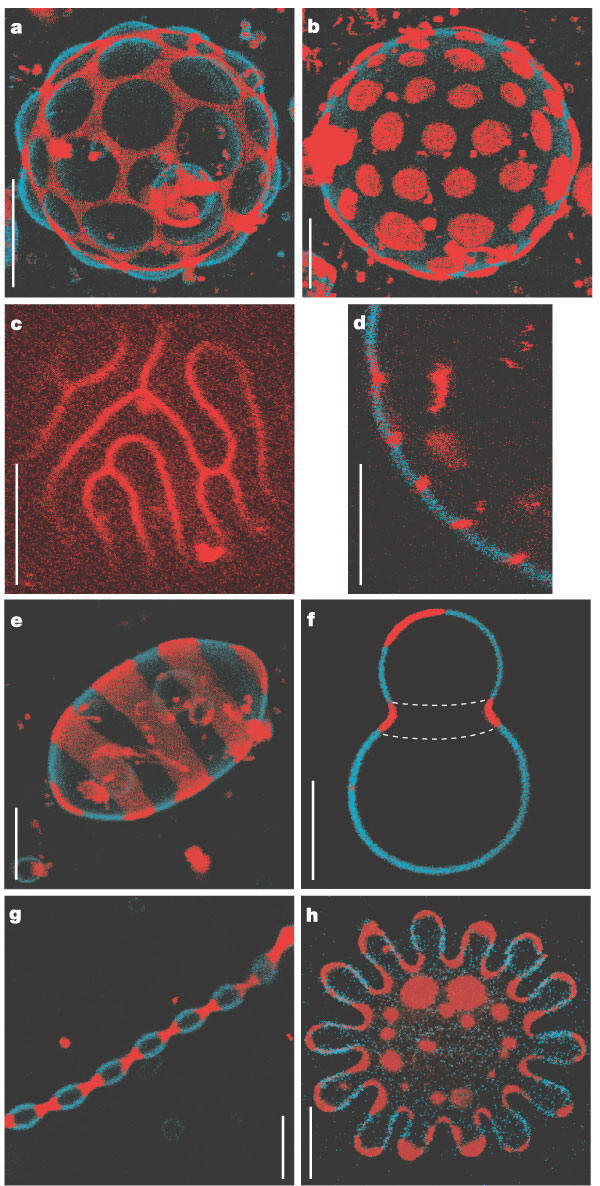
![]() Movie: Molecular Dynamics Simulation of vesicle fusion (scroll down to 52: Control of Membrane Fusion Reaction..)
Movie: Molecular Dynamics Simulation of vesicle fusion (scroll down to 52: Control of Membrane Fusion Reaction..)


