7: 7. Pollution, Overharvesting, Invasive Species, and Disease
- Page ID
- 63237
7. Pollution, Overharvesting, Invasive Species, and Disease
© 2019 J.W. Wilson and R.B. Primack, CC BY 4.0 https://doi.org/10.11647/OBP.0177.07

A pair of southern white rhinoceros (Ceratotherium simum simum, NT) in Tshukudu Private Game Reserve, adjacent to South Africa’s Kruger National Park. Once nearly extinct due to uncontrolled hunting, this species rebounded to over 20,000 individuals. Now, poaching is taking a toll again, even in premier protected areas such Kruger, where at least 662 rhinos were lost in 2016. Rangers have cut off the horn of one of the rhinos in the photo in a desperate attempt to prevent poaching. Photograph by Jan Fleishmann, https://commons.wikimedia.org/wiki/File:Nw_9302_white_rhinos_Tshukudu_JF.jpg, CC BY-SA 4.0.
Conservation biologists aim to preserve all the components, interactions, and processes within and between ecosystems, natural communities, species, and populations. The main obstacle to accomplishing this goal is habitat loss, while climate change will also play an increasingly important role. But let us for a moment consider widespread species and migratory populations. These species and populations typically live in different habitats and encounter different climates as they move across the landscape. We might think that tolerance for variety would make these groups robust against habitat loss and climate change. And yet, they are also declining, even in seemingly intact ecosystems and protected areas (Craigie et al., 2010; Lindsey et al., 2014). How can it be that populations apparently buffered from the two main extinction drivers are also subjected to population declines and extirpations?
Comprehensive conservation efforts must recognise that biodiversity faces multiple threats that need to be dealt with at different scales.
While habitat loss and climate change are the most prominent threats facing biodiversity at present, they are not alone. Nearly all human activities place additional pressures on populations, even those that already suffer from habitat loss and climate change. These additional pressures are primarily from pollution, overharvesting, persecution, invasive species, and disease (Maxwell et al., 2016). Because these threats are associated with and/or exacerbated by human activities, they can be dynamic in their nature, develop rapidly, and persist at such large scales that wildlife populations have little opportunity to adapt or move to safer areas. Moreover, these threats may interact with each other, as well as with climate change and habitat loss, so that their combined impact is greater than their individual effects. In this chapter, we explore how each of these threats impact wildlife and natural communities, and how they could push populations and species to extinction. Methods for lessening the impact of these threats are integrated into Chapters 9–15.
7.1 Pollution in Its Many Forms
Rachel Carson’s 1962 book, Silent Spring, described the dangers of pollution—pesticide pollution in particular—with a clarity that captured the public’s attention for many years afterwards. Carson, an American biologist, was particularly successful in drawing attention to biomagnification (also called bioaccumulation), a process through which pesticides and other toxins accumulate and become more concentrated in animals at higher levels of the food chain (Figure 7.1). Her work drew on research that found that dichlorodiphenyltrichloroethane (DDT), sprayed on crops to kill pest insects and on water bodies to kill malaria mosquito (Anopheles spp.) larvae, was also harming non-target organisms that consumed insects and fish exposed to DDT. Of note is that non-target organisms high on food chains, particularly fish-eating birds, such as eagles, pelicans, and egrets, often had high levels of DDT concentrated in their tissues. The affected birds were generally weakened, and the shells of their eggs were thin and prone to cracking during incubation. Consequently, bird populations declined dramatically in areas where DDT was used, as adults died and failed to raise young.

Figure 7.1 A simplified marine food web showing how sharks, marine mammals, seabirds, and even humans are all vulnerable to health problems associated with bioaccumulation where pesticides, heavy metals, and other harmful chemicals become concentrated at higher trophic levels. After Ross and Birnbaum, 2003, CC BY 4.0.
In the 1970s, many industrialised countries recognised the dire situation and banned the use of DDT, which eventually allowed for the partial recovery of the affected bird populations. Unfortunately, while some countries have switched to safer alternatives (e.g. Hargrove, 2003), DDT continues to be widely used in Africa to control malaria mosquito, tsetse fly (Glossina spp.), and other disease vectors. Researchers recently observed complete absences of breeding fish-eating birds in some African wetlands, and some of the highest-ever recorded DDT levels in seed-eating birds (Bouwman et al., 2013). This is cause for concern, not only for wildlife, but also for the long-term effects on people, particularly the consumers of the food products exposed to these chemicals (e.g. Manaca et al., 2011) and the workers who handle these chemicals in the field.
Pollution does not always lead to immediate mortality, but instead can have sublethal impacts that compromise organisms’ fitness over time, with population declines as the end result.
DDT is however not the only form of pollution we battle today. With the impacts of a growing human population becoming gradually more pervasive, pollution is compromising water, soil, and air quality at rates faster than ever before. Some forms of pollution can be highly visible, and with dramatic consequences (Figure 7.2). But importantly, there are many less detectable forms of pollution. While it may not always lead to immediate mortality, these insidious forms of pollution have sublethal impacts that compromise organisms’ fitness over time, with early death and population declines still being the end result. Responding to the silent threats of subtle and easily-overlooked pollution is often delayed, especially when the negative effects are felt only years after exposure. In their totality, pesticides and other pollutants claim 1.4–2.2 million human lives in Africa each year; globally, they claim 9 million lives, which is over three times more than the total impact of AIDS, malaria, and tuberculosis, together (Landrigan et al., 2018). Yet we continue to tolerate these threats, in part because the impact of pollution on our health is not always that apparent, especially when pollution deaths are expressed as a stroke, heart disease, respiratory infections, diarrhoea, or cancer, among other health issues.

Figure 7.2 A young boy next to an open sewer in Nairobi, Kenya. In addition to the dangers to human welfare and livelihoods, polluted waterways kill millions of native animals and plants each year, and harm countless number of ecosystems. Photograph by Eoghan Rice/Trócaire, https://commons.wikimedia.org/wiki/File:A_young_boy_sits_over_an_open_sewer_in_the_Kibera_slum,_Nairobi.jpg, CC BY 2.0.
One of the most challenging aspects when trying to prevent pollution is identifying the source. Many forms of pollution can easily be transported away from their source through the air, via rivers, even in groundwater. This transport of pollutants (called pesticide drift in the case of pesticides) means that a substantial burden (perhaps as much as 95%, Miller, 2004) of impacts are being felt by non-target species, including economically important non-target organisms. For example, pesticide drift from cotton fields in Benin has caused extirpations of freshwater fish (Agbohessi et al., 2015), while beneficial pollinating insects are also often negatively impacted (Pettis et al., 2013). Studies on fish in Nigeria (Adeogun et al., 2016), large mammals in South Africa (Bornman et al., 2010), and frogs in Kenya (Hayes and Menendez, 1999) have shown that beneficial organisms that survive this secondary pesticide exposure have disrupted reproductive and endocrine systems, and hence reduced fitness. Even humans may be exposed to secondary poisoning from pesticides, as toxic pesticide levels have been found in edible oysters and mussels in Ghana (Dodoo et al., 2013), prawn in Côte d’Ivoire (Roche and Tidou, 2009), and even chickens in South Africa (Barnhoorn et al., 2009).
To make matters worse, many pollutants take many years to biodegrade (i.e. break down in nature), and thus continue to pose a threat to wildlife and humans long after entering the environment. One important class of such long-lived pollutants is persistent organic pollutants (POP). Several types of pesticides qualify as POPs, which are prone to bioaccumulation and drift. The most famous POP is DDT; in the USA, biologist continue to see eggshell thinning and bird deaths, nearly 50 years after DDT was banned in that country (Burnett et al., 2013). This is a concern in places like Ethiopia’s Lake Koka, where recent studies have found DDT residues in every sample of fish tissue (from several different species) tested (Deribe et al., 2011). More information on POPs, many which are banned from use by signatories of the Stockholm Convention on Persistent Organic Pollutants, can be found on the Stockholm Convention website (http://pops.int).
There are also many types of persistent inorganic pollutants that find their way into the environment on a daily basis. One important class of persistent inorganic pollutants that also bioaccumulate is heavy metals; these include mercury, cobalt, copper, lead, and arsenic. A study from Zambia traced cobalt contamination in living trees to soil pollution from mining activities that occurred the mid-1970s (Mihaljevič et al., 2011). Some everyday products can also persist in the environment. For example, an aluminium can takes about 200 years to break down, while a plastic bag takes between 100–1,000 years to break down. The continued use of these products should thus raise alarm to anyone concerned about the environment and human health. But it also provides opportunities for any person to contribute to conservation by reducing use of these products and reusing/recycling those products that find their way into the supply chain.
Many pollutants take many years to biodegrade, and thus continue to pose a threat to wildlife and humans long after entering the environment.
7.1.1 Water pollution
Water pollution, the accidental or intentional dumping of pesticides; herbicides; oil products; fertilisers; sewage; industrial waste; detergents; and other foreign chemicals and objects into aquatic environments, is arguably the biggest current pollution concern in Africa (Prüss-Ustün et al., 2016; Landrigan et al., 2018).
The dumping of products containing heavy metals into aquatic environments is particularly concerning because heavy metals are toxic even in small concentrations, and likely to biomagnify. When aquatic organisms process contaminated water, they absorb or ingest the heavy metals along with other essential nutrients. With each additional step along the food chain, organisms ingest and accumulate increasingly higher concentrations of these toxic elements (see Figure 7.1). In this way, even small amounts of heavy metals can become lethal across several levels of the food web over time. Biomagnification is especially a concern with long-lived predatory marine fishes that people consumed as food, such as swordfish (Xiphias gladius, LC), marlins, sharks, and some tunas and sea basses. For example, mercury (emitted mainly during fossil fuel use), lead, and arsenic have bioaccumulated so much in sharks off South Africa that many species are now considered unsafe for human consumption (McKinney et al., 2016; Bosch et al., 2016; Merly et al., 2019). Recent studies also found unsafe levels of mercury in freshwater fish from regions as wide as Central Africa’s Great Lakes (Campbell et al., 2008), Ethiopia’s Lake Awassa (Desta et al., 2006), and several reservoirs in West Africa (Quédraogo and Amyot, 2013).
Because of biomagnification, many long-lived predatory marine fishes are now considered unsafe for human consumption.
Oil pollution involves the release of petroleum products into the environment, which can originate from damaged ships, failed drilling rigs, leaking offshore platforms, or other unexpected events. The released oil causes mammals and birds to lose the insulating abilities of their fur and feathers, leaving those animals vulnerable to hypothermia and drowning. Other aquatic animals, including fish and shellfish, may ingest oil products, causing them to sicken and die. Because of the way oil is extracted and transported, marine ecosystems are particularly at risk. Furthermore, because of the massive amount of oil that are involved in oil extraction and transport, an oil pollution event often represents a serious ecological disaster (Figure 7.3). Africa has been hit hard by oil spills in recent years, particularly around oil-producing countries like Angola and Nigeria, and along shipping lanes passing along the coasts of Namibia, South Africa, and Mozambique. Nigeria is perhaps the biggest victim of oil spills; between 1976 and 2001, there were an estimated 6,817 oil spills around Africa’s largest wetland, the Niger Delta (UNDP, 2006)! These oil spills have destroyed thousands of hectares of mangrove swamps, estuarine wetlands, and other coastal ecosystems, causing severe hardship to marginalised local communities who depended on those areas for subsistence fishing and farming (Fentiman and Zabbey, 2015).


Figure 7.3 (Top) Staff and volunteers from the seabird rescue centre SANCCOB are caring for some of the 19,000 African penguins (Spheniscus demersus, EN) that were rescued after a stricken iron ore carrier spilled 1,400 tonnes of oil off South Africa in June 2000 (Wolfaardt et al., 2009). (Bottom) SANCCOB volunteers releasing a group of African penguins that were rescued from the oil spill. Photographs by SANCCOB, CC BY 4.0.
Plastic pollution is fast becoming a ubiquitous threat to Africa’s environment, its wildlife, and its people. To visualise the magnitude of the problem, consider that there are more than 1.6 trillion pieces of plastic, collectively weighing over 70,000 tonnes, currently floating in the Atlantic and Indian Oceans surrounding Africa (Eriksen et al., 2014). While many of these plastic items were dumped directly in the ocean, many also have a terrestrial origin. For example, if someone throws a plastic wrapper on a sidewalk, there is a good chance that the wrapper will find its way into a nearby stream at some point, carried by wind or rain runoff. From here, the wrapper will float along various streams and rivers until it reaches the ocean. A recent review found that 88–95% of plastics floating into the world’s oceans originated from just 10 rivers, which include West Africa’s Niger River and East Africa’s Nile River (Schmidt et al., 2017; Lebreton et al., 2017). In the process, thousands of seabirds, dolphins, whales, turtles, seals and fish die each year from suffocation or starvation after ingesting plastics and other pieces of trash that they confused with food (Wilcox et al., 2015). This plastic pollution also impacts humans: researchers recently found microfibers (many of which are plastic) in over 80% of tap water samples from Uganda (Kosuth et al., 2017), as well as food-grade commercial sea salt originating from South Africa (Karami et al., 2017).
There are more than 1.6 trillion pieces of plastic, collectively weighing over 70,000 tonnes, currently floating in the Atlantic and Indian Oceans surrounding Africa.
Some of the biggest impacts from plastic pollution are caused not by visible scraps of plastic, but by microplastics, the collective name for plastic particles smaller than 1 mm (some are microscopic). Microplastics may originate from the breakdown of larger pieces of plastic and polystyrene products, or they may be manufactured intentionally small, such as beads added to cosmetics and other personal care products that are flushed down drains after use. Because microplastics are so small, they easily pass through the standard filters used at sewage treatment plants. Consequently, microplastics generally end up in the aquatic environment, where they are unintentionally consumed by crustaceans (crabs, lobsters, and krill), molluscs (mussels, oysters, and clams), echinoderms (sea stars, sea urchins, sea cucumbers), and baby fish. This consumption can block or damage the victim’s digestive and respiratory systems, cause reduced food uptake by creating a false sense of satiation, or even poison animals through leeching of synthetic chemicals. Each of these threats increases death rates and lowers reproductive rates (Sussarellu et al., 2016). Just as with the biomagnification we discussed earlier, the consumption of microplastics also affects other consumers (including humans), because the small organisms that ingest the microplastics are often food for other animals, allowing plastic pollution to move through an entire food chain. For example, a recent study from Lake Victoria found microplastics imbedded in the digestive tracts of perch and tilapia bought at a local market and meant for human consumption (Biginagwa et al., 2016). Because microplastics are so hard to remove once in an ecosystem, the best method for their containment may be to reduce plastic use, to ban products containing microplastics, or to develop microplastics that are biodegradable within a reasonable timeframe. But for this to happen, there is a need to educate the public and lawmakers (Galloway and Lewis, 2016) about the dangers posed by this threat to the environment and local economies.
Nutrient pollution, caused in part by excessive fertiliser use, can led to eutrophication, famous for causing algae blooms, aquatic dead zones, and fish kills.
Nutrient pollution represents another growing threat to Africa’s aquatic environments. Many lakes, streams, and other freshwater and marine environments naturally contain low concentrations of essential nutrients, such as nitrates and phosphates. In order to survive, the species living in these nutrient-poor waters must then be adapted to this natural nutrient scarcity. However, raw sewage, agricultural fertilisers, concentrated animal feeding operations, and industrial processes release large amounts of additional nitrates and phosphates into the environment, which are washed into the aquatic environment. Minor additions of essential nutrients stimulate plant growth, providing more food for organisms at higher trophic levels. However, at high concentrations, the system become subjected to nutrient pollution.
One of the worst outcomes of nutrient pollution is eutrophication. During eutrophication, surface algae grow so rapidly (known as an algae bloom) that it starts blocking sunlight from reaching aquatic organisms below the surface. Because each individual alga is short-lived, their rapid growth also adds large amounts of decaying matter to the environment. In response, decomposers that feed on the dead algae can become so abundant that they consume most of the water’s dissolved oxygen. Without oxygen and sunlight, aquatic plant and animal life may die off in large numbers. The resultant dead zones are sometimes visibly in the form of fish kills, with large numbers of dead fish floating on the surface of the affected water body. The organisms that die during this process is generally also toxic to humans because of bacteria build-up and other imbalances. Eutrophication is an increasingly common problem in Africa; for example, a recent review found that 41–76% of South Africa’s lakes may be eutrophic (Harding, 2015). Eutrophication has already negatively impacted Africa’s tourism and fisheries sectors (Nyenje et al., 2010), and even led a temporary shutdown of water supplies on the Kenyan side of Lake Victoria (Sitoki et al., 2012). Preventing further eutrophication should thus be a high priority—not only will it prevent harmful algae blooms but may even play an important role in controlling invasive aquatic plants such as the water hyacinth (Eichhornia crassipes) (Coetzee and Hill, 2012; Bownes et al., 2013).
Groundwater pollution—the release of pollutants into aquifers and other sources of groundwater—is also becoming a serious issue across Africa. This type of pollution generally originates from landfills, on-site sanitation systems, leaking sewage systems, mining leachate, agriculture runoff (fertiliser, pesticides, animal waste, etc.), and other types of waste dumping. The pollutants may sometimes be released directly into aquifers; however, more often the contaminants and pathogens leak into the soil, from where it seeps into groundwater.
Because fracking poses many serious risks, governments across the world have banned the practice from their lands.
One of the most important emerging threats to groundwater in Africa is hydrological fracturing or fracking, in short. During this process, pressurised liquids that contain suspended particles and thickening agents are blasted into rock formations deep underground to break them open. When the pressure and liquids are removed, the suspended particles keep the fractures open, which enables extraction of natural gas and petroleum. While fracking was initially hailed as a method to access previously inaccessible fossil fuels, scientists subsequently found that it poses a wide variety of very serious environmental and health risks. Most importantly, the liquids used in fracking contain toxic chemicals which pose a high risk for groundwater pollution (Osborne et al., 2011), which in turn lead to miscarriages and birth defects (McKenzie et al., 2014), cancer (McKenzie et al., 2012), as well as skin and respiratory diseases (Rabinowitz et al. 2015). In addition, fracking increases greenhouse gas emissions (Howarth, 2014) and induces infrastructure-damaging earthquakes (Ellsworth, 2013). Because of these myriad serious risks, several national governments in Europe, and several local governments in the USA, UK, Canada, and Australia have banned the practice from their lands (https://keeptapwatersafe.org/global-bans-on-fracking). In contrast, and despite opposition from civil society, several countries in Africa (e.g. South Africa: Roelf, 2016; Botswana: Barbee, 2015) recently approved this harmful practice.
7.1.2 Air pollution
In the past, people and industries thought that the atmosphere was so vast that any gases or particles released into the air would disperse and dilute to the point that they would post no ill effects. But as air quality has diminished over time, scientists have documented that air pollution can cause irreparable harm to ecosystems and human health, often far from the original sources. A striking example comes from West Africa’s Lake Chad, which shrank by 95% between 1963 and 1998 (Figure 7.4). Experts generally thought that the shrinkage was caused by unsustainable water use in the region, but recent evidence suggests that air pollution from Europe which reduced rainfall in the Lake’s catchment area may also have contributed to this ecological disaster (Hwang et al., 2013). The Lake’s water level has risen since 2007, likely due, in part, to clean air regulations implemented by the European Union. Despite this positive turn around, air pollution continues to be a serious problem (Amegah and Agyei-Mensah, 2017) that threatens humans and wildlife throughout Africa.

Figure 7.4 Changing rainfall patterns attributed to air pollution may have contributed to West Africa’s Lake Chad shrinking by 95% between 1963 and 1998. An ecological disaster ensued, as the 68 million people whose livelihoods were at risk put additional strains on the environment while trying to replace the natural resources the lake previously provided. Images by NASA/GSFC, https://svs.gsfc.nasa.gov/2065, CC BY 4.0.
An important form of air pollution is hydrocarbons, which are released during fossil fuel burning, particularly during transport, power generation, and other industrial activities (Karagulian et al., 2015). Pollution from airborne hydrocarbon compounds can sometimes be sensed without scientific equipment, by the bad smells, high air turbidity, and eye and lung irritation a person may experience in large cities with highly polluted air. When exposed to sunlight, these chemicals can react with other gases and particles in the atmosphere to produce photochemical smog, which is made up of ozone and other secondary compounds. In the upper atmosphere, ozone filters harmful ultraviolet radiation, which benefits most living things; but at ground level, high concentrations of ozone pose several dangers. For example, it damages plant tissues which make them brittle; high surface ozone levels have found to cause crop damage in Botswana and South Africa (Zunckel et al., 2004). Hydrocarbon exposure also poses several threats to humans: it altered some people’s DNA—often a cancer precursor—in Benin (Fanou et al., 2006), caused lung damage in Côte d’Ivoire (Kouassi et al., 2010), and subjected people to carcinogenic compounds in the DRC and Ghana (Tuakuila, 2013; Bortey-Sam et al., 2017). The lack of air monitoring and standards over much of Sub-Saharan (Petkova et al., 2013), and lack of awareness—people often confuse photochemical smog with natural mist and early-morning fog—should thus be of serious concern both to conservation biologists and society at large.
Air pollution from hydrocarbons often manifests itself as photochemical smog. Hanging like a thick cloud over industrial areas, people sometimes confuse it with natural mist and early-morning fog.
Burning fossil fuels also releases sulphur oxides (SOx) and nitrogen oxides (NOx) into the atmosphere, where they combine with water vapor to produce nitric and sulphuric acids. These acids later return to the ground as acid rain, with dramatically low pH relative to normal rainwater. Prevailing winds can transport acid rain clouds over long distances, so the effects of acid rain may occur hundreds of kilometres from its sources. Because the acid rain is closely tied to the water cycle, aquatic and soil organisms are particularly vulnerable to the negative effects of acid rain. Plants exposed to acid rain, either directly or after absorbing contaminated water from the ground, are often left severely weakened or even killed: it has even caused plant extirpations in Zambia (UNEP, 2006).
Another important contributor to air pollution is domestic fuel burning (Karagulian et al., 2015). During these activities, very small pollutant particles are released into the air. Because these particles are so small, they are difficult to filter from the air, and can easily be inhaled. Once inhaled, the particles can pass into the victim’s bloodstream, from where they negatively impact cardiovascular health, neurodevelopment, and cognitive function (WHO, 2013). Despite the harmful impact of these particles in the environment, their monitoring is virtually non-existent in Africa, making it very hard to guide air quality policy decisions and legislations. In contrast, measures that mitigate pollution from domestic fuel burning may even help slow the rate of habitat loss (Chapter 5), as this type of pollution is associated with inefficient wood stoves, slash-and-burn agriculture, and the artisanal charcoal industry.
7.1.3 Soil pollution
Soil pollution occurs when soil meets foreign chemicals and other pollutants. This type of pollution is often associated with industrial activities that extract resources from the earth, agricultural runoff, pesticide use, oil spills, acid rain, improper treatment of sewage, and improper disposal of waste. People and wildlife can then become sick through direct contact with contaminated soils, or through secondary contamination via polluted groundwater or eating food grown in contaminated soil. For example, a recent review reported how soil pollution has left medicinal plants toxic to humans in countries such as Botswana; Ghana; and Mali, at times with fatal consequences (Street, 2012).
The improper disposal of electronic waste (or e-waste in short) is a particularly serious form of soil pollution. Because electronic products contain toxic heavy metal contaminants that are expensive to recycle, discarded electronic products usually end up in dump yards (Figure 7.5). Here, open burning of electronic and other waste materials releases the toxic compounds into the soil, as well as the air and water (Robinson, 2009), from where it also accumulates in the environment.

Figure 7.5 Nearly all electronic goods contain parts with toxic chemicals that are expensive to recycle. Instead, such components end up in dump yards such as this one in Ghana, from where the toxic compounds pollute the air, water, and soil, posing many human and environmental risks. Photograph by Agbogbloshie Makerspace Platform, https://www.flickr.com/photos/qampnet/14937188796, CC BY-SA 2.0.
7.1.4 Light pollution
Light pollution describes the addition of excessive, ill-timed, or poorly designed artificial light to the natural world. A consequence of an increasingly industrialised world (Falchi et al., 2016), light pollution has increased dramatically over the past decades as more people have gained greater access to electricity (Figure 7.6). Behavioural disruption is perhaps the most well-known consequence of increased light pollution—consider all the moths and other nocturnal insects (and insect predators, such as bats and geckos) attracted to artificial night lights. Light pollution also interferes with the navigation abilities of nocturnal species, which often use the stars, moon, and light reflectance from water surfaces to orientate themselves. For example, work in Gabon has shown how artificial lights disorientate sea turtle hatchlings trying to reach the sea (Bourgeois et al., 2009), while others have highlighted the significance of light-induced seabird mortality (Black, 2005). These and other behavioural disruptions—which include attraction to and repelling away from artificial light—may seem to only affect a small number of individuals around a few lights in your home. But the systemic impact of thousands of lights every night has wide-ranging ecosystem impacts when considering the cumulative impact of reduced reproductive performance (Firebaugh and Haynes, 2016), disrupted predator-prey dynamics (Minnaar et al., 2015) and disturbed night-time pollination services (Knop et al., 2017) on the many thousands of organisms impacted every night.

Figure 7.6 Night-time composite of Africa and parts of Europe and Asia, taken by the Suomi NPP satellite in 2012. City lights of every major city in Africa can be seen; lights are particularly concentrated in South Africa and West Africa’s Gulf of Guinea. Image by NASA/GSFC, https://www.flickr.com/photos/gsfc/8246931247, CC BY 2.0.
Light pollution also disrupts the natural day-night cycles with which most species evolved. These disruptions interfere with circadian rhythms, which negatively affect living organisms’ physiology. For example, one study showed that night-time light pollution disrupted natural sleep patterns in birds, leaving the affected individuals more susceptible to malaria infections (Ouyang et al., 2017). Circadian rhythm disruptions from light pollution (especially from high frequency “blue” light) also impact humans by increasing stress, fatigue, and anxiety, and susceptibility to obesity (Rybnikova et al., 2016) and cancer (Haim and Portnov, 2013). It is important to note that light pollution does not mean that the use of light is inherently bad—light has and will continue to play an important role in our daily lives. However, it does mean that we need to be more thoughtful about the consequences of light pollution and put measures in place to mitigate its impacts on the natural world and our own lives.
7.1.5 Noise pollution
Many people find a sense of freedom when they are in natural surroundings, with peace and quiet facilitating a much-needed connection to nature. These experiences are increasingly being threatened by noise pollution. However, noise pollution (also called acoustic pollution)—caused by human activities, such as industrial, military, and transportation systems—affects more than just the appealing tranquillity of nature. It also prevents animals from hearing each other, predators, and prey, all which could interfere with feeding, reproduction, navigation, and predator-avoidance behaviours. While African studies on the impact of noise pollution on wildlife are near-absent (Shannon et al., 2015), one study that did investigate the topic found that traffic noise increased dwarf mongooses’ (Helogale parvula, LC) alertness but also reduced responsiveness to alarm calls (Kern and Radford, 2016). Such responses could leave the affected individuals less fit and more vulnerable to predators.
Noise pollution prevents interferes with communication, feeding, reproduction, navigation, and predator-avoidance behaviours; it may even contribute to mass strandings of whales.
One would think that marine organisms living in the vast oceans may be spared from noise pollution, but this is not the case (Koper and Plön, 2012; Kunc et al., 2017). Sound carries much further in salt water than air, so noises from ship propellers; military sonar; seismic activities, and construction have significantly increased the level of ambient noise levels marine organisms experience. This increased level of ambient noise not only disrupts communication in sea animals (e.g. Cerchio et al., 2014), but can even lead to death (some mass whale strandings have been attributed to noise pollution: Morell et al., 2017; Williams et al., 2017). As with light pollution, there is a general need to be more thoughtful about the consequences of sound pollution on the natural world and to put measures in place (see e.g. Koper and Plön, 2012) to mitigate its impacts.
7.1.6 Thermal pollution
Thermal pollution describes localised human-induced temperature changes to the natural world. Aquatic ecosystems represent one of the ecosystems most vulnerable to thermal pollution. For example, when water is released from big dams, it comes from the colder middle and lower strata of the reservoir, leading to rapid cooling of aquatic ecosystems further downstream. The opposite is true at power plants that use river water as a coolant; turbines release their heat to the circulating water and then the warmed water is released back into the environment. These abrupt releases of thermally discordant water often lead to thermal shock which can be lethal to fish and other aquatic organisms. For example, studies from South Africa have shown that thermal shock can kill fish embryos and larvae and caused deformities in the young of Clanwilliam yellowfish (Barbus capensis, VU) (King et al., 1998).
The urban heat island effect represents a terrestrial form of thermal pollution. Urban and other developed areas are generally covered with large swaths of man-made surfaces (e.g. asphalt roads, pavement surfaces, and building roofs), which absorb solar energy rather than reflect it. This absorbed heat, in combination with heat outputs from industrial activities, cause urban areas to function like “islands of heat” that are several degrees warmer (Figure 7.7) than surrounding rural areas (Feyisa et al., 2014; Chakraborty and Lee, 2018). The urban heat island effect reduces the quality of life for people and wildlife by reducing comfort and water availability (due to increasing evaporation). It also increases energy consumption to offset the heat increases which, in turn, contributes to air pollution and climate change.


Figure 7.7 (Top) Man-made surfaces such as roofs, roads, and pavements do not reflect, but rather absorb solar energy as heat, causing built-up areas to be warmer than the surrounding rural areas, CC BY 4.0. (Bottom) Data derived from 16-year mean daytime temperatures obtained by TERRA and AQUA satellites show how the urban heat island effect increases daytime temperatures of several African cities (represented as squares). Note how areas with high levels of deforestation (e.g. West Africa and Albertine Rift) also show the highest temperature increases (> 2.5°C). Map by T.C. Chakrabotry, after Chakrabotry and Lee, 2018, CC BY 4.0.
7.2 Overharvesting
People have always hunted, collected, trapped, or otherwise harvested the food and other natural resources they need to survive. When human populations were small, at least relative to the abundance of their resources, and collection methods were relatively unsophisticated, people could sustainably harvest and hunt wildlife in their local environments. However, as human populations have increased, and roads have provided access to previously remote areas, our impact on the environment has escalated. At the same time, our methods of harvesting have become dramatically more efficient. Guns are now used instead of blowpipes, spears, or arrows, while networks of wire snares indiscriminately catch animals of all types, even young and pregnant females. Populations of species that mature and reproduce rapidly can often recover quickly after harvests and can thus be exploited sustainably; however, species that are slow-maturing and slow-reproducing cannot sustain current harvest levels. Consequently, many species are threatened due to overharvesting, the unsustainable collection of natural resources (Maxwell et al., 2016). Overharvesting may take on many forms, including hunting, fishing, logging, and gathering of plants and animals for medicine, captive collections, subsistence, commerce, or recreation purposes (Figure 7.8).
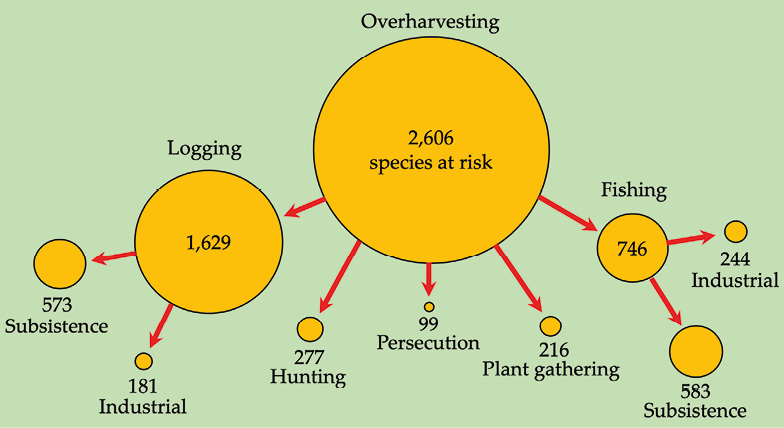
Figure 7.8 Overharvesting in Sub-Saharan Africa at scale: Over 60% of species that are threatened by overharvesting are also threatened (directly and indirectly) by logging. Nearly 30% of all species are threatened by fishing, and 10% by hunting and trapping. Source: IUCN, 2019, CC BY 4.0.
7.2.1 The Bushmeat Crisis
Bushmeat harvesting is one of Africa’s most prominent overharvesting concerns (see Box 4.1). Bushmeat—wild sources of protein obtained on land by hunting and collecting birds, mammals, snails, and caterpillars—provides much of the protein in people’s diets in large parts of Africa. For example, in Nigeria and Cameroon, 12,000 tonnes of bushmeat—two tonnes obtained from bay duiker (Cephalophus dorsalis, NT) alone—are sold at markets in the Cross-Sanaga rivers region each year (Fa et al., 2006). Similarly, more than 9,000 primates are killed annually for a single market in Côte d’Ivoire (Covey and McGraw, 2014); people from Central Africa harvest an astonishing 5.3 million tonnes of mammalian bushmeat annually (Fa et al., 2002). Usually seen as a conservation challenge in Africa’s tropical forests, the bushmeat crisis also impacts savannah regions (reviewed in Lindsey et al., 2013). For example, bushmeat hunters, numbering between 1.500 and 2,000, remove over 600,000 kg of herbivore biomass from Botswana’s Okavango Delta each year, despite the region’s protected status and importance for ecotourism sectors (Rogan et al., 2017).
The massive wildlife declines caused by the bushmeat crisis are also threatening ecosystem services, food security and people’s livelihoods.
Outside influences play a prominent role in the harvesting pressure associated with the bushmeat crisis. In Section 5.2, we discussed neocolonialism, where jobs associated with land-grabbing industries are frequently reserved for migrant labourers. Poorly paid and with limited rights, migrant labourers are often forced to turn to local natural resources to fulfil their basic needs (Thibault and Blaney, 2003). The impact of these migrant labourers on the local environment is massive compared to traditional (and many other local) peoples that prioritise sustainability. For example, immigrants working at logging concessions in the northern parts of the Republic of the Congo hunt 72% of all bushmeat harvested in the region (Poulsen et al., 2009). The increased commercialisation of bushmeat also poses challenges (Lindsey et al., 2013); for example, in the broader Congo Basin, commercial hunters are exploiting bushmeat at scales 27 times that of the area’s traditional peoples (Fa et al., 2016). In addition to hunting for local markets, illegal exports also play an important role. For example, more than 50 tonnes of wild fish and bushmeat enters France from Africa each week (Chaber et al., 2010); similar amounts were also estimated for airports in Switzerland (Wood et al., 2014).
Very few animal populations can withstand such high extraction rates. Consequently, regions dependent on bushmeat have already seen substantially wildlife declines (Lindsey et al., 2013). West Africa, where forest mammal populations are down an estimated 80%, have been hot particularly hard (Benítez-López et al., 2019). These wildlife declines also lead to reduced harvests—some hunters have seen their harvests reduced by over 80%, with impacts to wildlife notable as far as 40 km from hunters’ access points along roads from their villages (Benítez-López et al., 2017). At current exploitation rates, supplies are expected to decrease by an additional 80% within the next 50 years (Fa et al., 2003). Unless more sustainable, alternative sources of protein are found, people dependent upon bushmeat will see increased malnutrition and compromised livelihoods as bushmeat species are pushed to extinction. When that happens, families relying on bushmeat will face even worse food insecurity than that which is driving the current bushmeat crisis.
Exacerbating the risk of food insecurity, people in the affected regions will also suffer from compromised ecosystem services as populations of predators, seed dispersers, and pollinators are reduced (Rosin and Poulsen, 2016). For example, reduced mammal populations have been linked to reduced abundance of fruits and other useful plant products available for human consumption (Vanthomme et al., 2010). Some areas are already suffering from “empty forest syndrome”—a condition where a forest appears to be green and healthy, but is practically devoid of animals, and in which ecological processes have been irreversibly altered such that the forest’s species composition will change over subsequent decades (Nasi et al., 2011; Benítez-López et al., 2019). The bushmeat crisis is thus a major concern to people concerned about biodiversity and/or human well-being.
7.2.2 Overfishing
Pressure on biodiversity in aquatic environments is also increasing as people continue to harvest fish, sea turtles, dolphins, shellfish, and manatees for meat at increasing rates. Modernised fishing methods play a major role. For example, a motorised fishing fleet that faces few restrictions has caused a 75% decline in fish populations at Ethiopia’s Lake Tana in recent years (de Graaf et al., 2004). Also, in the marine environment, motorised fleets and enormous factory ships can now spend months at sea where they catch fish to sell at local and global markets (Ramos and Grémillet, 2013; Pauly et al., 2014). Some estimates suggest that wild-caught seafood could be virtually absent by 2050 if current exploitation levels persist (Worm et al., 2006).
For many aquatic organisms, the indirect impacts of modern commercial fishing methods outweigh direct exploitation (Figure 7.9). One example is ghost fishing, which causes thousands of animals to die each year after becoming entangled in dumped, abandoned, and lost fishing gear. Similarly, approximately 25% of fish harvests are considered bycatch—animals that are accidentally caught, injured, or killed during fishing operations. Recent declines in skates, rays, turtles, sharks, dolphins, and seabirds have all been linked to incidental deaths as bycatch (Cox et al., 2007; Carruthers et al., 2009). Seabird biologists from South Africa have been at the forefront of solving bycatch problems in recent years (Box 7.1).
Box 7.1 Solving Seabird Bycatch Problems: From Theory to Practice
DST-NRF Centre of Excellence at the FitzPatrick Institute of African Ornithology,
University of Cape Town, South Africa.
rosswanless@gmail.com
The global problem of seabird bycatch in fisheries—the accidental death of seabirds during fishing—is one of the biggest threats to pelagic seabirds (Croxall et al., 2012). Ironically, it is both one of the easiest and one of the most challenging problems to solve. How so? Simple technical fixes to stop birds from getting snagged on fishing gear and drowning can work amazingly well, but fishermen must be convinced to use them.
Techniques for preventing bycatch break down into two basic approaches. The first approach is to prevent access to the danger point (the baited hook or the cables that birds strike). Fishing only at night eliminates up to 80% of the problem, but still jeopardises nocturnal foragers and diurnal species during a full moon. Another option is bird-scaring lines (Figure 7.A) consisting of a mainline flown from the stern of a boat with hanging streamers that scare birds away from danger areas behind the vessel. The second approach, primarily used in longline fishing, is to remove the risky gear (baited hooks) as quickly as possible; essentially this involves adding weights to lines to sink them faster. It has reduced seabird bycatch on some fisheries by 90–95%.

Figure 7.A Bird-scaring lines (in orange) keep a variety of albatross, petrels, and gannets at a safe distance from the baited hooks deployed off a trawler off South Africa. Photograph by BirdLife South Africa Albatross Task Force Programme, CC BY 4.0.
Despite clear harm to seabirds caused by fisheries and the simple, effective fixes at hand, implementing these mitigation measures has been patchy at best in most fisheries where seabird bycatch occurs. There are some exceptions, and it is useful to examine what elements led certain fisheries to fix the problem. A good case study is in South Africa, where BirdLife South Africa’s Albatross Task Force (ATF) demonstrated in 2006 that trawl fishing for hake (Merluccius spp.) was killing around 18,000 seabirds each year (Watkins et al., 2008). The fishery involved had Marine Stewardship Council (MSC) certification, which gives a fishery access to premium European markets on the condition that it meets environmentally friendly and sustainable metrics, including no significant bycatch impacts. This provided a powerful incentive for fleet-wide implementation of a bycatch mitigation measure; failure to do so would have resulted in a loss of certification, with catastrophic financial implications.
Despite this strong incentive, it required another five years of work from the ATF to assess bird scaring lines and refine the design, overcome resistance to their use, and close loopholes in regulations. In 2014, the ATF published an assessment of the effectiveness of their bird scaring lines—a single measure to prevent the accidental and avoidable deaths of around 10,000 albatrosses and large numbers of other species. When used correctly, the system eliminated 90–95% of seabird bycatch (Maree et al., 2014). Why did it take so long for the fleet to adopt this measure, despite it costing almost nothing, requiring no skill or time to use, and posing no meaningful operational problems? And why have identical fishing industries in many other countries failed to follow suite?
The answer is complex. ATF teams are present in South Africa (and now also in Namibia), providing sustained pressure and constant presence. South Africa had standing legislation, yet compliance from the South African fleet was initially minimal (and remains less than perfect today). MSC certification certainly created an enabling environment (Wanless and Maree, 2014) and incentive to drive change, yet it took more than that to change the entire fleet. Constant lobbying from BirdLife and regular dialogue from deck to boardroom were also critical ingredients. A legislative framework that provides some hope of censure against non-compliant vessels meant that there was internal pressure within the industry to “tow the line”—pun intended. Ultimately, widespread change became possible when there was a credible, independent observer programme to verify deck practice and give teeth to agencies when addressing non-compliance.


Figure 7.9 (Top) Discarded fishing gear, such as this ghost net, poses an entanglement hazard to marine wildlife. Photograph by Tim Sheerman-Chase, https://www.flickr.com/photos/tim_uk/ 2692835363, CC BY 2.0. (Bottom) A wandering albatross (Diomedea exulans, VU) that was a victim of bycatch, the accidental catching of non-target species during fishing operations. Photograph by Graham Robertson, CC BY 4.0.
7.2.3 The impact of traditional medicine
Africa has a long history of sustainable use of traditional medicines. Unfortunately, as the number of people living in Africa has increased, so has the demand for traditional medicine. Today, harvesting for traditional medicine is putting unsustainable pressure on species exploited for this purpose (Williams et al., 2014). One prominent example is vultures: the demand of vulture body parts, believed to bestow clairvoyant abilities, is driving massive vulture population declines across Africa (see Box 4.4). The growth in traditional medicine markets in East Asian countries such as China, Thailand, Cambodia, and Vietnam exacerbates these problems. For example, as tigers (Panthera tigris, EN) and rhinoceros have become scarce in Asia, Asian traditional healers are increasingly targeting African predators and rhinoceros to satisfy their market demands. Another group of species threatened by the Asian traditional medicine trade is sea horses (Hippocampus spp.). Due to population declines from overharvesting, sea horse exports from Kenya and Tanzania to East Asia have halved over recent years; yet, more than 600 kg of dried sea horses (over 254,000 individuals) continue to be exported annually (McPherson and Vincent, 2004). Exploitation for Asian traditional medicine markets has already pushed the western black rhinoceros (Diceros bicornis longipes, EX) to extinction. In a similarly perilous position is the northern white rhinoceros (Ceratotherium simum cottoni, CR); with only two non-reproductive females left in the world, this species is now considered committed to extinction (see Section 8.3; Box 11.4). A group of species sought after by both African and Asian traditional medicine markets is pangolins, thought to be the most heavily poached animals on Earth. For example, between 2012 and 2016, more than 20 tonnes of African pangolin scales (involving up to 30,000 animals) were seized during law enforcement operations across the region (Andersen, 2016). The problem is also getting worse: authorities intercepted 13 tonnes of scales in Singapore in 2019, all from a single shipment believed to travel from Nigeria to Vietnam (Geddie, 2019). With such a large active operational scale, it comes as no surprise that all four African pangolin species are now threatened with extinction (IUCN, 2019).
7.2.4 The impact of live animal trade
Millions of non-domesticated animals are sold as pets around the world each year (Table 7.1). Given that many of these pets were originally collected in the wild, it is no surprise that the most popular species tend to be at a high risk of extinction (Bush et al., 2014). These huge numbers are magnified by the extra millions of animals needed to compensate for deaths during collection and shipping. Collection of wild animals for pets and other purposes has a massive impact of biodiversity in Africa, the world’s largest pet trade exporter (Bush et al. 2014).
Among the most popular groups of wildlife traded are Africa’s parrots (Figure 7.10). For example, 32,000 wild-sourced African grey parrots (Psittacus erithacus, EN) were imported into the European Union in 2005 (UNEP-WCMC, 2007). Combined with habitat loss, the wild bird trade has already caused extirpations of this species in some areas of West Africa (Annorbah et al., 2015). Similarly, 82 of the 291 species of African freshwater fish known to occur in the pet trade are considered threatened with extinction (UNEP-WCMC, 2008). While it is true that collecting wild animals for the pet trade sustains many people’s livelihoods, research on harvesting of ornamental fish in Cameroon has shown that this practice is not sustainable in the long term (Brummet et al., 2010). It is therefore critical to find ways to make these practices more sustainable, for the sake of the pet collectors and biodiversity.
Table 7.1 Examples of groups targeted in global wildlife trade, and their levels of exploitation.
|
Group |
Number traded each year |
Notes |
|
Orchids |
250 million |
Mainly cultivated, but about 10% sourced from the wild. Illegal trade—and mislabelling to avoid regulation—a major problem. |
|
Succulent plants |
35 million |
Mainly cultivated, but about 15% sourced from the wild. Illegal trade remains a major problem. |
|
Corals |
13 million |
Collected using destructive methods; used for aquarium decor and jewellery. |
|
Reptiles |
7.2 million |
Mainly sourced from the wild for zoos and pet trade, but increasingly from farms. Does not include large skin trade. |
|
Birds |
2.3 million |
Mostly perching birds destined for zoos and pet trade. Also includes legal and illegal trade of parrots. |
|
Ornamental fish |
2 million |
Most originate from wild reefs, caught by illegal methods that damage the surrounding coral reef and other wildlife. |
|
Primates |
148,000 |
Used for biomedical research, while many also destined for pets, circuses, zoos, and private collections. |
Sources: http://cites-dashboards.unep-wcmc.org, data presented as live specimens exported from 2011–2015. Data generally do not include illegal traded specimens, which are usually not reported to CITES.

Figure 7.10 Wild-caught African grey parrots crammed into a travel crate before export to Asia. Researchers estimate that over 60% of smuggled parrots die from stress, dehydration, and smothering during transit (Mcgowan, 2008). Because of trade-driven population declines, CITES banned all international trade in this species in October 2016. Photograph by Lwiro Primates, CC BY 4.0.
7.2.5 Overharvesting of plant products
Overharvesting is not restricted to animals and animal products. While legal and illegal timber and firewood extraction is a major source of deforestation throughout Africa, it is also an important extinction driver. In fact, logging and other forms of wood harvesting have already contributed to the extinction of at least six plant species in Sub-Saharan Africa, with an additional 116 species considered Critically Endangered in part due to these threats (IUCN, 2019). Other plant species face extinction due to exploitation for medicines, spices, fragrances, and ornaments. For example, White’s ginger (Mondia whitei)—reputed to have aphrodisiac and antidepressant properties—has been harvested to extirpation in parts of central Kenya and South Africa (Aremu et al., 2011). Similarly, harvesting rates of African blackwood (Dalbergia melanoxylon, NT)—popular for making musical instruments and fine furniture—are currently unsustainable because the tree is slow-growing, has low germination rates, and extractions are seldom offset with planting of new seeds or seedlings (Amri et al., 2009).
7.2.6 Challenges in managing overharvesting
One of the biggest challenges in combatting overharvesting is the non-enforcement and/or outright absence of legal controls to protect exploited species. But even where strong regulatory frameworks exist, the sheer scale of the problem poses practical challenges for effective enforcement (discussed in Chapter 12), as billions of dollars flow among participants in illegal wildlife trade, which include local people trying to make a living, professional poachers, corrupt government officials, unethical dealers, and wealthy buyers who are not concerned about how the wildlife products they use were obtained. The illegal wildlife trade has hit Africa’s megafauna particularly hard. For example, even though there has been an international ban on the ivory trade since 1989, thousands of African elephants continue to be illegally killed on an annual basis (Box 7.2). Similarly, despite a ban on rhinoceros horn trade since 1977, an increasing number of rhinoceros succumb to poaching every year (Figure 7.11). Worse yet, the illegal wildlife trade shares many characteristics and practices with the illegal trade in drugs and weapons; in some cases, the same syndicates run these various criminal enterprises (Christy and Stirton, 2015). Apprehending these criminal networks is generally very dangerous, requiring vast resources.
Box 7.2 Conserving Elephants in the Anthropocene
1FitzPatrick Institute of African Ornithology, University of Cape Town,
Cape Town, South Africa.
2Tropical Resource Ecology Programme, University of Zimbabwe,
Harare, Zimbabwe.
cummingdhm@gmail.com
As our increasingly human-dominated planet enters a new geological era, will there still be room for Earth’s largest land mammals? Or will there be, as happened to mammoths, sabre-toothed cats, and giant sloths during the Pleistocene (see Box 8.1), another hominid-induced extinction of large mammals? Our new human-dominated era has become known as the Anthropocene (Waters et al., 2015), and the animals are, of course, Africa’s elephants.
Elephants encapsulate the dilemmas of conserving large charismatic mammals. They are dominant ecosystem engineers that, depending on their densities, can facilitate or adversely impact species diversity and ecosystem processes (Section 4.2.1). They are also economically important to ecotourism industries and revered by many; ivory ornaments and carvings have been valued highly by many cultures past and present. But elephants are also regarded as dangerous pests by expanding small-scale farming communities, responsible for destroying crops and killing people. While retaliatory killings and habitat loss (primarily through agricultural expansion) certainly contribute to the endangerment of elephants, poaching to supply Asian markets (see Figure 12.1) is the primary cause behind massive population reductions we are currently witnessing (Wittemyer et al., 2014).
Africa’s elephants have, in the past, been greatly exploited, first for their meat and later also for their ivory. In 1887, about 1,000 tonnes of ivory were being exported from Africa (Spinage, 1973) and, by 1900, elephant populations in many African countries had all but collapsed. In Southern Africa, for example, it was feared that they might soon go extinct. However, with effective protection, elephant populations increased twentyfold, to more than 200,000, south of the Zambezi River by the 1970s. Elsewhere, elephant numbers also recovered, and, in the mid-1970s, the continental elephant population was estimated to be more than 1 million (Table 7.B). But a rapid escalation of the illegal killing of elephants for ivory and meat soon followed, accompanied by a steep rise in the price of ivory. In response, African elephants were placed on CITES Appendix II in 1976 to control the international trade in ivory. Elephants in some Southern African countries were well protected, so numbers continued to grow. However, elsewhere poaching and illegal trade in ivory continued and, in 1989, the African elephant was placed on CITES Appendix I, which banned all international trade in elephants and elephant products. The result was a decline in the price of ivory, and recovery of many populations.
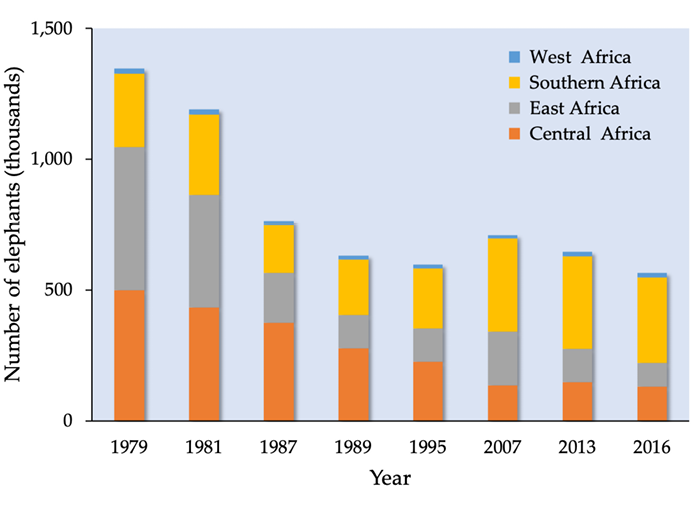
Figure 7.B Estimated number of elephants in West, Central, East and Southern Africa between 1979 and 2016. Source: http://africanelephantdatabase.org, CC BY 4.0.
Conservationists, and the world at large, traditionally regarded the African elephant as a single species. Recent morphometric and genetic evidence has revealed that forest and savannah elephants represent two distinct species, with forest elephants (Loxodonta cyclotis) occupying the West and Central African forests, and the larger savannah elephants (Loxodonta africana) being widespread in non-forested regions of Sub-Saharan Africa (Roca et al., 2015). The distinction has important implications for their conservation, as each of these elephant species now viewed on its own is even more sensitive to population declines (CBD, 2015).
Since about 2006, poaching of elephants again began to escalate, in part a response to an increase in the price of ivory, poorly funded wildlife agencies, and corruption (Hauenstein et al., 2019). The scale of these killings is extraordinary. For example, an estimated 30,000 elephants were killed in 2013 alone. Forest elephants declined by about 60% (Maisel et al., 2013). While population trends for savannah elephants vary across the region, they too face increased poaching pressure (Chase et al., 2016). Due to these large-scale killings, combined with the impact of habitat loss from agricultural expansion, West Africa’s elephants are today confined to small isolated protected areas with a total population of about 17,000 (Maisel et al., 2013). Elephant population trends in East Africa vary: numbers are increasing in Uganda and Kenya, but Tanzania has lost some 60,000 elephants in the last few years. In Southern Africa, Botswana has the largest elephant population, estimated in 2014 to number at least 130,000. Neighbouring Zimbabwe has a population of 83,000 elephants, much the same number as it had in 2001. However, two of Zimbabwe’s four regional populations declined significantly between 2006 and 2014 with a loss of at least 20,000 elephants (Figure 7.C).

Figure 7.C Savannah elephants at a waterhole in Hwange National Park. Hwange, Zimbabwe’s flagship protected area, has been a hotspot of poacher activity in recent years. By lacing waterholes and salt licks with cyanide during the dry season, poachers killed over 100 elephants here in 2013 (Cruise, 2017); several elephant poisoning events have occurred since then. Photograph by D.H.M. Cumming, CC BY 4.0.
Global and national efforts to curb elephant poaching are currently focused on improving law enforcement on the ground, intercepting ivory shipments to Asia, closing ivory markets in Africa and Asia, and leading campaigns to reduce demand for ivory in major consuming countries in Asia, particularly in China. Importantly, while these initiatives are relieving poaching pressure on African elephant populations, they fail to address core issues relating to the interactions between people and elephants in the rural areas of Africa. A high proportion of elephant ranges lie outside protected areas where they overlap with people. Relieving continued pressures on elephants, outside as well as inside protected areas, would only happen if people who are harmed by elephants derive enough benefits from elephants and other wildlife to outweigh the direct and indirect costs of sharing land with them. Community-based natural resource management (CBNRM) projects, such as those in Namibia (Section 14.3) show that this can be achieved. Establishing secure and sustainable funding streams through payments for ecosystem services (Section 15.3), or payments for co-existing with large dangerous mammals such as elephants (Section 14.4), could extend these promising initiatives even further.

Figure 7.11 More than 7,200 rhinoceros were illegally killed in South Africa between 2007 and 2017. Fortunately, illegal killings have declined in recent years, thanks to massive anti-poaching campaigns and increased law enforcement efforts. Source: SADEA, CC BY 4.0.
Now, consider a hypothetical conservationist concerned about the bushmeat crisis’ impact on biodiversity. This person may very well think that effective enforcement of a hunting ban would be the best solution to prevent further overharvesting. Unfortunately, solving complex challenges with simplistic steps runs a high risk of being counterproductive. For example, local bushmeat markets provide important contributions to food and financial security in many rural parts of Africa (van der Merwe et al., 2015). Replacing bushmeat with livestock and crops production—two primary drivers of habitat loss (Chapter 5)—also carries risks. For example, an estimated 250,000 km2 of forest will need to be converted to pastureland to replace the bushmeat trade just for the Congo Basin (Nasi et al., 2011). Clearly, there is a need for the bushmeat trade, albeit in a sustainable manner to ensure long-term viability of the local biodiversity, as well as the prosperity of the people who inhabit these areas. We delve deeper into solutions for these kinds of complex challenges from Chapter 9 onwards.
7.3 Persecution
Persecution involves the indiscriminate abuse or killing of a group of animals, generally used as a strategy to prevent property damage (e.g. crop-raiding elephants) and livestock depredation (e.g. lions endangering cattle). Some animals are also persecuted because of the real or perceived dangers they pose to humans; this includes the indiscriminate killing of sharks, snakes, and spiders to avoid bites, and culling of bats to prevent spread of zoonotic diseases (Schneeberger and Voigt, 2016). Lastly, local folklore also contributes to persecution: animals, such as moles, chameleons, and owls are sometimes indiscriminately killed because of cultural beliefs that they bring bad luck.
While killing a (potential or perceived) problem animal may bring a certain instant gratification, it is a short-term solution that often causes more harm than good.
While killing a (potential or perceived) problem animal may bring a certain instant gratification, it is a short-term solution that often causes more harm than good. Culling bats, for example, may in fact increase the prevalence of the same zoonotic diseases people are trying to control (Schneeberger and Voigt, 2016). Persecution also leads to the loss of ecosystem services, as it often targets ecosystem engineers and keystone species (Section 4.2.1). Retaliatory poisoning is particularly harmful for all the other useful organisms that may be killed in the process. One study in Namibia estimated that about 100 non-target animals are killed for every target animal, putting harmless species such as aardwolf (Proteles cristatus, LC), bat-eared fox (Otocyon megalotis, LC), and Cape fox (Vulpes chama, LC) also at risk (Brown, 2006). Among the most vulnerable to such poisoning are vultures, which may be accidentally poisoned by bait set out for problem predators, or directly targeted for traditional medicine and to hide poaching activities. In June 2019, 537 vultures (comprising five different Endangered and Critically Endangered species) were killed in Botswana after scavenging from three poisoned elephant carcass (de Greef, 2019). This mass poisoning event was particularly devastating because it was during the vulture breeding season, so many vulture chicks likely also died from starvation if not from eating tainted meat brought back to the nest by their parents.
7.4 Invasive Species
An exotic species is a species that occurs beyond its native range, most often because humans have moved it, whether intentionally or not. Most exotic species do not establish viable populations in the new areas to which they have been moved because the new environments may not meet their needs, because native species may outcompete them or otherwise displace them, or because there may not be a sufficient number of individuals to become established. However, a small number of exotic species go on to become invasive species—exotic species that rapidly spread and increase in abundance at the expense of native species and ecosystems. While there is no definitive list of qualities that predict which exotic species can become invasive, many invasive species have the following in common: (1) they begin to reproduce at an early age; (2) they can reproduce rapidly; (3) they lack sufficient predators in their introduced range; (4) they disperse easily; (5) and they are generalist species, able to survive in a variety of ecosystems.
Invasive species displace native species through competition, predation, and habitat alterations. They often thrive in environments disturbed by human activities.
7.4.1 Spread of invasive species
As stated above, invasive species spread and invade new areas because human activities move them there. Some of the most prominent means by which human activities facilitate the spread of invasive species include:
- Agriculture: Large industries exist to grow agricultural plants for crop production, ornamental plants for gardens, grasses for pastures, and livestock for food. Many of these organisms later escape from cultivation and captivity and go on to invade and harm local ecosystems. Other species spread when industry workers accidentally harvest the seeds of weedy plants along with commercial seeds, and then sow those seeds in new localities, while microbes, parasitic organisms, and insects may be transported with plant leaves and roots, in potting soil, or even attached to transported animals. One of Africa’s worst plant invaders, the triffid weed (Chromolaena odorata), continues to spread because of its sustained use to boost soil fertility on agricultural lands (Uyi et al., 2014).
- Accidental transport: Many invasive species spread to new areas because people transported them unintentionally. Domestic rats (Rattus spp.) and mice (Mus spp.) are classic examples: they have spread around the world as stowaways aboard ships (Box 7.3). A great number of invasive plants that currently occur in South Africa’s Cape Floristic Province arrived by accident by clinging onto the luggage and hiking gear of tourists (Anderson et al., 2015). The ballast tanks of ships are also common hiding places for invasive aquatic species: DNA analyses have shown how a German ship carried tiny snails in this way along the entire length of Africa’s Atlantic coast, providing invasion opportunities across this entire shipping route (Ardura et al., 2015).
- Biological control: Environmental and agricultural organisations sometimes use biological control (Section 4.2.7) to manage the spread of, and harm caused by, invasive species. While this approach can be very effective, in rare cases the biocontrol agent can become invasive and harm native species, rather than its intended target. For example, domestic cats that were introduced to Marion Island off Africa’s south coast feasted on native seabirds—in some cases, even causing seabird extirpations—instead of the rats and mice they were meant to control (Bloomer and Bester, 1992). For this reason, very careful research is necessary to test the appropriateness of a biocontrol agent before being released.
- Deliberate introductions: Soon after their arrival, colonists released hundreds of European birds and mammals into countries like South Africa and Kenya to make the African countryside feel more familiar. Other species, especially fish (e.g. trout, bass, and carp), were released to provide food and recreational opportunities. Many of these species have subsequently become so successful that they harm native species. For example, mesquite (Prosopis juliflora), introduced to Ethiopia from Mexico to reduce soil salinity, proved such a successful invader that it completely displaced local plants; the subsequent encroachment even threatens the viability of Ethiopia’s few remaining Grevy’s zebras (Equus grevyi, EN) (Kebede and Coppock, 2015). The introduction of the Nile perch (Lates niloticus, LC) to the Rift Valley to boost local fisheries likely led to the extinction of hundreds of fish species endemic in Lake Victoria (Pringle, 2005).
- Captive escapees: Many invasive species were originally kept as pets or ornamental plants but have escaped from captivity to establish feral populations that harm local wildlife. Some of the most problematic aquatic invasive species are common pets that people dumped in streams, lakes, or storm drains because they could not care for them anymore. Finding these escapees before they establish should thus be a priority. For example, a recent survey found that 258 alien ornamental plant species growing in South Africa’s Kruger National Park are at risk of becoming invasive—most of these plants were subsequently removed from the park (Foxcroft et al., 2008).
Box 7.3 Aliens on Islands: Damage and Control
FitzPatrick Institute of African Ornithology, DST-NRF Centre of Excellence,
University of Cape Town, South Africa.
peter.ryan@uct.ac.za
Invasive species are one of the main threats to biodiversity. Island ecosystems are particularly vulnerable; of the 156 bird species that have gone extinct in the last 500 years, more than 90% lived on oceanic islands (IUCN, 2019). This vulnerability is mainly due to species on oceanic islands evolving in the absence of competing species or predators, and thus lacking adequate defences against introduced species (including humans).
Invasive species pose many threats to island biodiversity. Newly arrived mammalian predators have exacted the greatest toll. Seemingly unable to appreciate the danger posed by these strange new arrivals, the ecologically-naïve adult birds simply remain on their nests to be eaten rather than fleeing. Introduced herbivores can have devastating impacts, because many island plants lack defences like tough leaves or thorns. Most devastating are domestic goats and rabbits, introduced by early island explorers to provide a source of food in the case of shipwreck, that have grazed many once-lush islands down to the ground. Introduced plants can also outcompete native plants. For example, the Mexican thorn (also called mesquite, Prosopis juliflora) has formed dense thickets on the once sparsely vegetated lowlands of Ascensión Island, making those areas unsuitable for both nesting seabirds and sea turtles.
Some of Africa’s least-transformed islands are the sub-Antarctic Prince Edward Islands, 2,000 km southeast of Cape Agulhas, and Gough Island, 2,800 km west of Cape Town. Their small size, isolation, and lack of sheltered harbours prevented human settlement. Nevertheless, their large seal and seabird populations were frequently exploited for oil and skins in the 19th and early 20th centuries. In the 19th century, sealing parties accidentally introduced house mice (Mus musculus) to Marion Island, the larger of the two Prince Edward Islands, and Gough Island. The mice flourished by eating native invertebrates and plants, probably causing the local extinction of one flightless moth on Marion Island. A few domestic cats were brought in to control the mice at Marion Island’s weather station, established in 1948. Instead, the cats targeted the island’s birds, which were easier prey. By the 1970s, some 2,000 cats were killing an estimated 450,000 seabirds each year, greatly reducing the island’s burrow-nesting petrels and even driving some species to local extinction (Bloomer and Bester, 1992). The events on Marion contrasted with nearby predator-free Prince Edward Island that continued to support vast breeding populations of burrowing petrels.
A pioneering initiative eradicated Marion Island’s cats in 1991, using a combination of introduced cat influenza, hunting, trapping, and poisoning (Bloomer and Bester, 1992). Researchers hoped Marion’s seabird populations would recover within a decade but had not considered the impact of mice once the cats were removed. The precedent was set on Gough Island; in 2001, introduced mice were discovered to predate on large numbers of seabird chicks, including Tristan albatross (Diomedea dabbenena, CR) chicks more than 100 times larger than themselves (Davies et al., 2015; Dilley et al., 2015a). It was hypothesised that mice are more likely to attack seabirds when they are the sole introduced predators on an island. Sure enough, the first attacks on Marion’s albatross chicks were recorded in 2003 (Figure 7.D); by 2015, the attacks had increased dramatically (Dilley et al., 2015b).

Figure 7.D Two grey-headed albatross fledglings (Thalassarche chrysostoma, EN), still on the nest, suffering from injuries after predatory mouse attacks on Marion Island, southern Indian Ocean. The bird in the foreground already has its scalp exposed. Attacks usually occur at night; mice will come back to the attack site over successive nights until the victims succumb to their injuries. Photograph by P.G. Ryan, CC BY 4.0.
Fortunately, it is possible to eradicate invasive species from islands. In 2014, Australia removed mice, rats, and rabbits from sub-Antarctic Macquarie Island (Parks and Wildlife Service, 2014), which is almost twice the size of Gough Island. Plans are now also in place to eradicate Gough’s mice in 2019. The island’s isolation facilitates this effort—damage to other species can be minimized, and possible spread of toxins or diseases will be confined. To access areas inaccessible on foot, helicopters will be used to spread poison bait from specially designed hoppers slung under the aircraft. Some poisoning of non-target native individuals is inevitable, but this is a small price to pay compared to extinctions of those species. If adequate measures are put in place to prevent subsequent reintroductions, there is hope of restoring at least part of the island’s natural balance.
7.4.2 Impact of invasive species
Invasive species have many negative consequences for native biodiversity: they displace native species through competition, alter the structure and composition of natural communities, and sometimes also hybridise with native species. These impacts may also translate to financial losses, as invasive species compromise ecosystem services (Figure 7.12), damage infrastructure, and spread infectious diseases.

Figure 7.12 The tickberry (Lantana camara) may be pretty, but it is a serious invasive species across Africa. Where it invades, it reduces the productivity of natural ecosystems and agricultural lands by forming dense thickets that outcompete native plants. This plant is also toxic to animals, including livestock and pets. Photograph by Alves Gaspar, https://en.wikipedia.org/wiki/File:LantanaFlowerLeaves-3.jpg, CC BY-SA 3.0.
Invasive species often become pervasive because they outcompete and displace native species. One such example is the Mediterranean mussel (Mytilus galloprovincialis), which was accidentally introduced to South Africa in the mid-1970s via European ships. A superior competitor, the exotic mussel soon started displacing native mussels and limpets, especially in the inter-tidal zone of South Africa’s west coast (Branch and Steffani, 2004). Considered South Africa’s most successful marine invasive, recent evidence suggests that the Mediterranean mussel is continuing to spread north into Namibia and along South Africa’s east coast towards Mozambique.
Another superior competitor is the water hyacinth. A native to South America’s Amazon forest, this species was intentionally introduced as a showy ornamental plant to dams, ponds, and lakes across Africa in the early 20th century. The plant established well, but then started reproducing and spreading at such rapid rates that water bodies across the region were soon covered by a dense mat of leaves. With little surface exposure and water movement, eutrophication and suffocation followed, leading to the deaths of countless fish and other aquatic organisms (Villamagna and Murphy, 2010). A biological control program targeting hyacinth showed promise during the 1990s; however, eutrophication from fertiliser overuse (which stimulate growth of hyacinth and other invasive aquatic plants) may be contributing to this species’ recent resurgence (Coetzee and Hill, 2012; Bownes et al., 2013).
A single gum or pine tree can transpire as much as 50,000 litres of water per year, while plantations of these trees can reduce water resources in an area by as much as 70%.
Natural communities are at particular risk in cases where invasive species change ecosystem structure and functioning so much that native species can no longer survive. Such is the case across many parts of Africa, where invasive Australian gum (Eucalyptus spp.) and pine (Pinus spp.) trees (both widely planted for timber) transpire so much water through their leaves (as much as 50,000 litres of water per tree/year; Dzikiti et al., 2016) that they can reduce the availability of surface- and groundwater in an area by as much as 70% (le Maitre et al., 2016). In addition to creating drought conditions, the closed canopies created by these invasive trees reduce the amount of solar radiation reaching the ground, greatly limiting thermoregulatory opportunities for taxa such as reptiles (Schreuder and Clusella-Trullas, 2016). Habitat degradation caused by invasions of the cinnamon tree (Cinnamomum verum), originally from Sri Lanka, has already caused at least ten invertebrate extinctions in the Seychelles (IUCN, 2019).
While many of the invasive species mentioned earlier originated from outside Africa, it is important to note that African species can also become invasive in other parts of Africa when they are moved outside of their native ranges. When invading nearby areas, non-native species can come in close contact with closely-related species, creating a high risk of genetic mixing—also called genetic pollution or genetic swamping—which describes the hybridisation of invasive species with native species. For example, hybridisation with the widespread banded tilapia (Tilapia sparmanii, LC) threatens the survival of Namibia’s Otjikoto tilapia (Tilapia guinasana, CR), globally restricted to the < 1 km2 Lake Guineas (Bills 2007). The Cape platanna (Xenopus gilli, EN), an endemic to South Africa’s Cape Floristic Region, is similarly threatened by hybridisation with the widespread African clawed frog (Xenopus laevis, LC) (Fogell et al., 2013).
7.4.3 Genetically modified organisms
A topic of conflict among conservation biologists is the increased popularity of genetically modified organisms (GMO). A GMO is an organism whose genetic material has been altered to provide useful or improved products and services. To do this, scientists typically use genome editing technologies to transfer genes from a “source” organism into the DNA of the target organism. For example, scientists can transfer a bacterial gene that produces an insect toxin into a crop, such as maize, to obtain a GMO that can resist insect herbivory. Farmers using this GMO maize would then be able to increase production and reduce pesticide use (Gewin, 2003). While GMOs are usually associated with the development of pest-resistant and drought-resistant crops, uses are highly varied. For example, in Senegal, GMO technologies are used to produce tilapia that are better adapted to local ecosystems (Eknath et al., 2007). GMO technologies are being used to develop new and cheaper medicines (Concha et al., 2017), and to combat important diseases: trials in Burkina Faso shows that fungi genetically engineered to produce spider toxins caused a 99% collapse in malaria-carrying Anopheles mosquito populations within 45 days (Lovett et al., 2019). GMOs can even be used for conservation purposes, like developing new methods to combat invasive species (Esvelt et al., 2014), creating more effective bioenergy sources (Beer et al., 2009), and making vulnerable species more resistant to climate change (Piaggio et al., 2017). Some scientists even hope to combat plastic pollution by creating a genetically modified bacterium able to consume plastic waste (Austin et al., 2018).
The use of GMOs is not a new phenomenon. Selective breeding, hybridisation, and other forms of artificial selection—techniques that have been used for much of human history—all result in different forms of genetically modified crops and animal species. However, technological advances in genetic engineering have enabled scientists to transfer genes from and between different taxa that have not previously been used in selective breeding programmes (i.e. viruses, bacteria, insects, fungi, and shellfish). GMO technologies that transfer genetic material between wholly disparate taxa has led to concern about the unknown and unintended consequences of such “crossovers”. Some people are also concerned that GMOs that escape from captivity or cultivation (e.g. Gilbert, 2010) could hybridise with closely-related wild species, endangering native wildlife while resulting in new, aggressive weeds and virulent diseases. Additionally, the use of GMO crops could potentially harm non-crop species (e.g. insects, birds, and soil organisms) that live in, on, or near the GMO crops. Concerns have also been raised about the potential effects to people eating GMO foods, leading some governments to regulate GMO research and commercial applications differently than traditional agriculture. However, after decades of research, it appears that GMO food is safe to eat (Blancke, 2015).
GMOs offer benefits such as increased production and reduced pesticide use, but there are concerns about unknown and unintended consequences like hybridisation and invasiveness.
Clearly, GMOs offer a wide range of opportunities which could directly and indirectly benefit biodiversity conservation. However, the benefits of GMOs must be examined and weighed against the potential risks on a case by case basis. It is probably wise to proceed cautiously, to study GMOs thoroughly, and to monitor their impacts on ecosystems and human health in the areas where they are used. These investigations could involve workshops where experts come together to perform environmental impact assessments (EIA) (Section 12.2.2). The potential but unknown impacts of GMOs can also be mitigated by limiting the ability of these organisms to spread or reproduce (Muir and Howard, 2004).
7.6 Parasites and Diseases
Parasites and diseases have always been an important natural factor in regulating the ecology of wildlife, especially in wild populations that have become unsustainably large. Today, however, human activities are facilitating increased spread and transmission of parasites (Box 7.4) and other pathogens, sometimes even creating conditions for epidemics to develop (Figure 7.13). Consequently, parasites and diseases have become a major threat to wildlife, including those already suffering under low population sizes and densities.
Box 7.4 Promoting African and Global Honeybee Health
Agroscope, Swiss Bee Research Center,
Schwarzenburgstrasse, Switzerland.
vincent.dietemann@alp.admin.ch
Modern society imposes increased pressure on animals and plants to secure the food needed for a growing human population. Honeybees especially contribute to crop productivity in a crucial way thanks to their pollination services. Unfortunately, the number of bee colony losses has surged in recent years in several regions of the world (Goulson et al., 2015), worrying scientists, politicians, and the public. Some regions in Africa, however, have maintained healthy domesticated and wild honeybee colonies (Pirk et al., 2016), which continue to pollinate flowers and enhance the production of many fruit and vegetable crops.
The power of comparisons
Comparative studies have always been important to biological research. Studying a model organism or system under different conditions allows scientists to identify how these organisms or systems react and adapt to their environment. This can even be done at the continental scale and could help us understand the effect of human pressure exerted on honeybees. In general, beekeeping in North America and Europe has been widely industrialised, involving large-scale operations and modern technology, whereas in Africa beekeeping has remained small-scale and mostly low-tech. This gives us an opportunity to determine the effects of beekeeping management and trade on honeybee health.
Different contexts
The varroa mite (Varroa destructor) originally parasitised the eastern honeybee (Apis cerana). In the wake of global honeybee trade, this parasite has invaded most regions of the world that are home to the western honeybee (Apis mellifera) and resulted in major colony losses (Figure 7.E). Although eastern and western honeybees are closely-related species, the western honeybee did not coevolve with this parasite and, thus, has few natural defences against it, with colonies dying within a few years after infestation. Consequently, only those colonies treated against the parasite by beekeepers can survive, and most wild honeybee populations have been decimated. However, there have been exceptions: colonies of the western honeybee in the southern parts of Africa are resistant to the parasite, and large wild populations remain. Several international teams have now turned their attention to resistant African honeybee populations to understand the basis of their survival (Strauss et al., 2016). Researchers hope to use this knowledge to promote the breeding of surviving colonies in currently susceptible populations both within and outside of Africa.

Figure 7.E During its development, this young honey bee worker has been damaged by the varroa mite it carries on the front end of its abdomen. Its deformed wings are a consequence of this parasitism. Photograph by Vincent Dietemann, CC BY 4.0.
Africa also differs strongly in land use and crop management techniques that are likely to influence honeybees’ nutrition and health. In many areas, small-scale farming prevails, with a lower use of pesticides than in other areas of the world. Understanding how pesticide and other chemical use, as well as how nectar and pollen variety and quality, impact these pollinators will be key to their survival. Therefore, the rest of the world may learn how to maintain healthy honeybees from Africa. Africa, in return, might benefit from global efforts to maintain sustainable pollination services and promote food security.

Figure 7.13 Habitat loss and encroachment of human activities into natural areas increase the rates of transmission of infectious diseases such as influenza, rabies, canine distemper virus, and Ebola between wildlife, domestic animals, and human. This figure illustrates the infection and transmission routes of rabies. Blue arrows represent factors that leads to higher infection rates, while red allows represent factors that contribute to the spread of the disease among the three vulnerable groups. After Daszak et al., 2000, CC BY 4.0.
One way in which humans elevate the impact of parasites and diseases on wildlife is by exposing native species to harmful organisms that they have never previously encountered, and thus have no evolved coping mechanisms. For example, population declines and extirpations of about 200 frog species across the world, including in Africa (Tarrant et al., 2013; Hirschfeld et al., 2016), is due, in part, to a disease caused by the chytrid fungus (Batrachochytrium dendrobatidis). This disease, known as chytridiomycosis (Figure 7.14), affects a frog’s ability to absorb water and electrolytes through the skin (Alroy, 2015). It likely originated in the Korean Peninsula (O’Hanlon et al., 2018), and spread across the world through trade with African clawed frogs (Xenopus laevis, LC) (Weldon et al., 2004). As of yet, there is no cure for this disease, and it continues to be seen as one of the biggest threats currently facing the world’s amphibians.

Figure 7.14 Biologists swabbing an olive striped frog (Phlyctimantis leonardi, LC) in Gabon to screen for the chytrid fungus. Frogs from Cameroon has tested positive (Baláž et al., 2012) which suggests the species might be at risk; however, thus far no ill effects have been observed. Photograph by Brian Gratwicke, https://www.flickr.com/photos/briangratwicke/4395505435, CC BY 2.0.
Disease transmissions can also occur when humans and their pets or livestock interact with wildlife (Cumming and Cumming, 2015). For example, during the early 1990s about 25% of lions in Tanzania’s Serengeti National Park were killed by canine distemper virus which they contracted from domestic dogs living near the park (Kissui and Packer, 2004). Because of the many biological similarities between apes and humans, gorillas (Gorilla spp.), chimpanzees (Pan troglodytes, EN) and bonobos (P. paniscus, EN) are particularly vulnerable to anthroponotic diseases, such as measles, influenza, and pneumonia which can be transferred from humans to animals. But even chytridiomycosis (discussed above) can become an anthroponotic disease, transferred from frog to frog by a careless biologist that handles a healthy frog after a sick one without taking precautions against transmission. Some diseases (e.g. Ebola; flu; and tuberculosis) can be anthroponotic and zoonotic (transferred from animals to humans). While the impact of Ebola on humans in Africa is well-known, it is worth noting that gorillas suffer > 90% mortality when exposed to Ebola, compared to 50% mortality in humans. In fact, it was an Ebola outbreak in 2004 that caused the western lowland gorilla (Gorilla gorilla gorilla, CR) to be classified as highly threatened by the IUCN (Genton et al., 2012).
Human activities often facilitate the emergence and spread of infectious diseases, which threaten wildlife, domestic species, and humans alike.
Humans also indirectly facilitate the transmission and spread of parasites and pathogens. While there are some exceptions (notably social insects), transmission and infection rates are typically low for wildlife living in large, complex ecosystems because they have space to move away from disease-carrying droppings, saliva, old skin, and other sources of infection. However, these natural buffers against pathogens and parasites are removed when humans confine those organisms to small areas (such as small fenced reserves) or keep them in crowded conditions. In addition to forcing those organisms to remain in close contact with potential sources of infection, crowded conditions lead to deterioration of habitat quality and food availability. Both these factors increase the organisms’ stress levels and reduce their body conditions which, in turn, lowers their resistance to parasites and diseases (reviewed in Gottdenker et al., 2014).
Human-induced extirpations indirectly facilitate the transmission and spread of parasites and pathogens, even to humans. Such is the case with schistosomiasis (also known as bilharzia), a zoonotic disease carried by a few freshwater snail species. In the 1980s, health care professionals observed an increased incidence of human schistosomiasis around Lake Malawi after overfishing depleted snail-eating fish populations, followed by decreased incidence of schistosomiasis as fish populations recovered in the 1990s (Stauffer et al., 2006). A similar situation occurred in East Africa, where the elimination of apex predators resulted in increased olive baboon (Papio anubis, LC) populations, which not only worsened crop raiding, but also increased parasite infection rates among local peoples (Brashares et al., 2010).
Parasites and diseases also threaten captive wildlife populations, including those kept at zoos and other ex situ conservation facilities (Section 11.5). Because of the proximity in which different species are kept, captive conditions may allow for easier spread of diseases. An added complication with captive populations is that some individuals may function as disease reservoirs. These individuals generally appear healthy because they are fairly resistant to the disease they carry, yet they are able to infect other susceptible individuals. Disease reservoirs frequently limit opportunities for translocation of captive populations (Section 11.2), even when dealing with threatened species. For example, well-meaning people often bring raggedy-looking yet healthy penguins in moult to rehabilitation centres, hoping the penguins will be released once “better”. Yet, those animals might never be released back in the wild to avoid the risk of transmitting diseases to wild penguin populations (Brossy et al., 1999).
The impacts of diseases are bound to become more important in the future of conservation biology, especially as growing human populations and increased competition for space increase the need for single-species management and ex situ conservation (Chapter 11). Disease management should therefore always be taken very seriously, and appropriate steps taken to avoid disease transmissions.
7.7 Summary
- Many threats to biodiversity do not lead to immediate and/or direct mortality, but instead have sublethal impacts that compromise organisms’ fitness over time. Responses to these silent, insidious, and easily-overlooked threats are often delayed, especially when the negative effects are felt only years after exposure.
- Environmental pollution leaves ecosystems uninhabitable for native wildlife, and cause sickness and death in wildlife and people. Common causes of pollution include pesticides, heavy metals, plastic, fossil fuels, fertilisers, light, heat, and noise, leading to pollution of water, groundwater, air, and soil.
- Overharvesting is becoming an increasingly damaging threat to biodiversity because people have better access to previously unexploited areas and are adopting increasingly efficient methods for harvesting wildlife products. Persecution, which has its roots in human-wildlife conflict, is becoming an important threat because a growing human population is increasingly encroaching on the shrinking remaining natural habitats.
- Invasive species outcompete local species and change the structure and composition of their native ecosystems. Human activity is responsible for these invasions, by accidentally or deliberately moving wildlife to new regions of the world. Some invasive species require a great amount of effort and resources to manage.
- Disease transmission and spread increase when wildlife is confined to small areas and/or crowded conditions. Diseases may also be transmitted between wildlife, domesticated species, and even humans. Managing for diseases is also important in zoos and other ex situ facilities, because diseases spreading from one individual to another can prevent those individuals from being released into the wild.
7.8 Topics for Discussion
- Which forms of environmental pollution are most prominent in the region where you live? Which natural ecosystems are impacted most severely by this pollution? How are humans affected? What do you think can be done to reduce or even eliminate this pollution?
- Consider all the fishing, hunting, trapping, collecting, logging, and other wildlife harvesting activities in your region. Which activities are well managed, and which are not? Why is it so difficult to regulate these activities, when so many people know that overharvesting would eventually harm local economies, their families, and their livelihoods? What measures do you think can be put in place to control overharvesting in your region, or at least reduce its impact?
- Briefly describe what biological control is, and what its benefits are. What are the risks involved in biological control? How can these risks be predicted and avoided?
- Can you name a few diseases that can be transmitted from people to wildlife, and from wildlife to people? Which species are involved in each of these diseases? How does the transmission of each of these diseases impact the conservation management of species involved?
7.9 Suggested Readings
Benítez-López, A., L. Santini, A.M. Schipper, et al. 2019. Intact but empty forests? Patterns of hunting-induced mammal defaunation in the tropics. PLoS ONE 17: e3000247. https://doi.org/10.1371/journal.pbio.3000247 African mammals are undergoing catastrophic declines due to unregulated hunting.
Bouwman, H., I.M. Viljoen, L.P. Quinn, et al. 2008. Halogenated pollutants in terrestrial and aquatic bird eggs: Converging patterns of pollutant profiles and impacts and risks from high level. Environmental Research 126: 240–52. https://doi.org/10.1016/j.envres.2013.06.003 DDT threaten African biodiversity and people even today
Campbell, L., P. Verburg, D.G. Dixon, et al. 2008. Mercury biomagnification in the food web of Lake Tanganyika (Tanzania, East Africa). Science of The Total Environment 402: 184–91. https://doi.org/10.1016/j.scitotenv.2008.04.017 Biomagnification of heavy metals are making some fish unsafe to eat.
Galloway, T.S., and C.N. Lewis. 2016. Marine microplastics spell big problems for future generations. Proceedings of the National Academy of Sciences 113: 2331–33. https://doi.org/10.1073/pnas.1600715113 The threat of plastic pollution to ocean life.
Gottdenker, N.L., D.G. Streicker, C.L. Faust, et al. 2014. Anthropogenic land use change and infectious diseases: A review of the evidence. EcoHealth 11: 619632. https://doi.org/10.1007/s10393-014-0941-z Linking environmental degradation to disease risk in humans and wildlife.
le Maitre, D.C., G.G. Forsyth, S. Dzikiti, et al. 2016. Estimates of the impacts of invasive alien plants on water flows in South Africa. Water SA 42: 659–72. http://dx.doi.org/10.4314/wsa.v42i4.17 The impact of invasive plants on water cycles
Maxwell, S.L., R.A. Fuller, T.M. Brooks, et al. 2016. The ravages of guns, nets and bulldozers. Nature 536: 143–45. https://doi.org/10.1038/536143a Biodiversity faces many anthropogenic threats.
Pringle, R.M. 2005. The origins of the Nile perch in Lake Victoria. BioScience 55: 780–87. https://doi.org/10.1641/0006-3568(2005)055[0780:TOOTNP]2.0.CO;2 The devastating impact of invasive fish in Lake Victoria.
Stauffer, J.R., H. Madsen, K. McKaye, et al. 2006. Schistosomiasis in Lake Malawi: Relationship of fish and intermediate host density to prevalence of human infection. EcoHealth 3: 22-27. https://doi.org/10.1007/s10393-005-0007-3 Trophic cascades can even affect human health.
Bibliography
Adeogun, A.O., O.R. Ibor, S.D. Adeduntan, et al. 2016. Intersex and alterations in reproductive development of a cichlid, Tilapia guineensis, from a municipal domestic water supply lake (Eleyele) in southwestern Nigeria. Science of the Total Environment 541: 372–82. https://doi.org/10.1016/j.scitotenv.2015.09.061
Agbohessi, P.T., I.I. Toko, A. Ouédraogo, et al. 2015. Assessment of the health status of wild fish inhabiting a cotton basin heavily impacted by pesticides in Benin (West Africa). Science of the Total Environment 506: 567–84. https://doi.org/10.1016/j.scitotenv.2014.11.047
Alroy, J. 2015. Current extinction rates of reptiles and amphibians. Proceedings of the National Academy of Sciences 112: 13003–08. https://doi.org/10.1073/pnas.1508681112
Amegah, A.K., and S. Agyei-Mensah. 2017. Urban air pollution in Sub-Saharan Africa: Time for action. Environmental Pollution 220: 738–43. https://doi.org/10.1016/j.envpol.2016.09.042
Amri, E., Z.L. Kanyeka, H.V.M. Lyaruu, et al. 2009. Evaluation of genetic diversity in Dalbergia elanoxylon populations using random amplified polymorphic DNA markers. Research Journal of Cell and Molecular Biology 3: 71–79. https://doi.org/10.15406/mojbm.2017.01.00015
Andersen, I. 2016. Seizure of huge African pangolin scale shipment points to worrying increase in trafficking (Gland: IUCN). https://www.iucn.org/news/secretariat/201606/seizure-huge-african-pangolin-scale-shipment-points-worrying-increase-trafficking
Anderson L.G., S. Rocliffe, N.R. Haddaway, et al. 2015. The role of tourism and recreation in the spread of non-native species: A systematic review and meta-analysis. PLoS ONE 10: e0140833. https://doi.org/10.1371/journal.pone.0140833
Annorbah, N.N.D., N.J. Collar, and S.J. Marsden. 2016. Trade and habitat change virtually eliminate the Grey Parrot Psittacus erithacus from Ghana. Ibis 158: 82–91. https://doi.org/10.1111/ibi.12332
Ardura, A., A. Zaiko, J.L. Martinez, et al. 2015. Environmental DNA evidence of transfer of North Sea molluscs across tropical waters through ballast water. Journal of Molluscan Studies 81: 495–501. https://doi.org/10.1093/mollus/eyv022
Aremu, A.O., L. Cheesman, J.F. Finnie, et al. 2011. Mondia whitei (Apocynaceae): A review of its biological activities, conservation strategies and economic potential. South African Journal of Botany 77: 960–71. https://doi.org/10.1016/j.sajb.2011.06.010
Austin, H.P., M.D. Allen, B.S. Donohoe, et al. 2018. Characterization and engineering of a plastic-degrading aromatic polyesterase. Proceedings of the National Academy of Sciences 2018: 201718804. https://doi.org/10.1073/pnas.1718804115
Baláž, V., O. Kopecký, and V. Gvoždík. 2012. Presence of the amphibian chytrid pathogen confirmed in Cameroon. Herpetological Journal 22: 191–94.
Barbee, J. 2015. Botswana sells fracking rights in national park. Guardian. https://gu.com/p/4eck4
Barnhoorn, I.E.J., M.S. Bornman, C. Jansen van Rensburg, et al. 2009. DDT residues in water, sediment, domestic and indigenous biota from a currently DDT-sprayed area. Chemosphere 77: 1236–41. https://doi.org/10.1016/j.chemosphere.2009.08.045
Beer, L.L., E.S. Boyd, J.W. Peters, et al. 2009. Engineering algae for biohydrogen and biofuel production. Current Opinion in Biotechnology 20: 264–71. https://doi.org/10.1016/j.copbio.2009.06.002
Benítez-López, A., L. Santini, A.M. Schipper, et al. 2019. Intact but empty forests? Patterns of hunting-induced mammal defaunation in the tropics. PLoS ONE 17: e3000247. https://doi.org/10.1371/journal.pbio.3000247
Benítez-López, A., R. Alkemade, A.M. Schipper, et al. 2017. The impact of hunting on tropical mammal and bird populations. Science 356: 180–83. https://doi.org/10.1126/science.aaj1891
Biginagwa, F.J., B.S. Mayoma, Y. Shashoua, et al. 2016. First evidence of microplastics in the African Great Lakes: Recovery from Lake Victoria Nile perch and Nile tilapia. Journal of Great Lakes Research 42: 146–49. https://doi.org/10.1016/j.jglr.2015.10.012
Bills, R. 2007. Tilapia guinasana. The IUCN Red List of Threatened Species 2007: e.T63354A12662434. http://doi.org/10.2305/IUCN.UK.2007.RLTS.T63354A12662434.en
Black, A. 2005. Light induced seabird mortality on vessels operating in the Southern Ocean: Incidents and mitigation measures. Antarctic Science 17: 67–68. https://doi.org/10.1017/S0954102005002439
Blancke, S. 2015. Why people oppose GMOs even though science says they are safe. Scientific American. https://www.scientificamerican.com/article/why-people-oppose-gmos-even-though-science-says-they-are-safe
Bloomer, J.P., and M.N. Bester. 1992. Control of feral cats on sub-Antarctic Marion Island, Indian Ocean. Biological Conservation 60: 211–19. https://doi.org/10.1016/0006-3207(92)91253-O
Bornman, M.S., I.E.J. Barnhoorn, C. de Jager, et al. 2010. Testicular microlithiasis and neoplastic lesions in wild eland (Tragelaphus oryx): possible effects of exposure to environmental pollutants? Environmental Research 110: 327–33. https://doi.org/10.1016/j.envres.2010.02.003
Bortey-Sam, N., Y. Ikenaka, O. Akoto, et al. 2017. Oxidative stress and respiratory symptoms due to human exposure to polycyclic aromatic hydrocarbons (PAHs) in Kumasi, Ghana. Environmental Pollution 228: 311–20. https://doi.org/10.1016/j.envpol.2017.05.036
Bosch, A.C., B. O’Neill, G.O. Sigge, et al. 2016. Heavy metal accumulation and toxicity in smoothhound (Mustelus mustelus) shark from Langebaan Lagoon, South Africa. Food Chemistry 190: 871–78. https://doi.org/10.1016/j.foodchem.2015.06.034
Bourgeois, S., E. Gilot-Fromont, A. Viallefont, et al. 2009. Influence of artificial lights, logs and erosion on leatherback sea turtle hatchling orientation at Pongara National Park, Gabon. Biological Conservation 142: 85–93. https://doi.org/10.1016/j.biocon.2008.09.028
Bouwman, H., I.M. Viljoen, L.P. Quinn, et al. 2008. Halogenated pollutants in terrestrial and aquatic bird eggs: Converging patterns of pollutant profiles and impacts and risks from high level. Environmental Research 126: 240–52. https://doi.org/10.1016/j.envres.2013.06.003
Bownes, A., M.P. Hill, and M.J. Byrne. 2013. The role of nutrients in the responses of water hyacinth, Eichhornia crassipes (Pontederiaceae) to herbivory by a grasshopper Cornops aquaticum Brüner (Orthoptera: Acrididae). Biological Control 67: 555–62. https://doi.org/10.1016/j.biocontrol.2013.07.022
Branch, G.M., and C.N. Steffani. 2004. Can we predict the effects of alien species? A case-history of the invasion of South Africa by Mytilus galloprovincialis (Lamarck). Journal of Experimental Marine Biology and Ecology 300: 189–215. https://doi.org/10.1016/j.jembe.2003.12.007
Brashares J.S., C.W. Epps, and C.J. Stoner. 2010. Ecological and conservation implications of mesopredator release. In: Trophic Cascades, ed. by J. Terborgh and J. Estes (Washington: Island Press).
Brossy, J.J., A.L. Plös, J.M. Blackbeard, et al. 1999. Diseases acquired by captive penguins: What happens when they are released into the wild? Marine Ornithology 27: 185–86.
Brown, C.J. 2006. Historic distribution of large mammals in the Greater Fish River Canyon Complex, southern Namibia, and recommendations for re-introductions (Windhoek: Namibia Nature Foundation). http://www.the-eis.com/data/literature/Greater%20Fish%20River%20Canyon%20 Complex%20Historic%20distribution%20of%20mammals.pdf
Brummet, R.E., C. Cargill, L.M. Lekunze, et al. 2010. Stream degradation, fish abundance and the potential viability of ornamental fisheries in south-western Cameroon. African Journal of Aquatic Science 35: 155–64. https://doi.org/10.2989/16085914.2010.497650
Burnett, L.J., K.J. Sorenson, J. Brandt, et al. 2013. Eggshell thinning and depressed hatching success of California Condors reintroduced to central California. Condor 115: 477–91. https://doi.org/10.1525/cond.2013.110150
Bush, E.R., S.E. Baker, and D.W. Macdonald. 2014. Global trade in exotic pets 2006–2012. Conservation Biology 28: 663–76. https://doi.org/10.1111/cobi.12240
Campbell, L., P. Verburg, D.G. Dixon, et al. 2008. Mercury biomagnification in the food web of Lake Tanganyika (Tanzania, East Africa). Science of The Total Environment 402: 184–91. https://doi.org/10.1016/j.scitotenv.2008.04.017
Cannon, J.C. 2018. Half a ton of pangolin scales seized on the way to Asia from Benin. Mongabay. https://news.mongabay.com/2018/04/half-a-ton-of-pangolin-scales-seized-on-the-way-to-asia-from-benin
Carruthers, E.H., D.C. Schneider, and J.D. Neilson. 2009. Estimating the odds of survival and identifying mitigation opportunities for common bycatch in pelagic longline fisheries. Biological Conservation 142: 2620–30. https://doi.org/10.1016/j.biocon.2009.06.010
Carson, R. 1962. Silent Spring (Boston: Houghton Mifflin).
CBD (Center for Biological Diversity). 2015. Petition to reclassify and uplist African elephants from Threatened to Endangered under the Endangered Species Act as two separate species: Forest elephants (Loxodonta cyclotis) and savannah elephants (Loxodonta africana) (Tucson: Center for Biological Diversity). https://www.biologicaldiversity.org/species/mammals/pdfs/African_Elephant_Uplisting_Petition.pdf
Cerchio, S., S. Strindberg, T. Collins, et al. 2014. Seismic surveys negatively affect humpback whale singing activity off northern Angola. PloS ONE 9: e86464. https://doi.org/10.1371/journal.pone.0086464
Chaber, A.-L., A. Allebone-Webb, Y. Lignereux, et al. 2010. The scale of illegal meat importation from Africa to Europe via Paris. Conservation Letters 3: 317–21. https://doi.org/10.1111/j.1755-263X.2010.00121.x
Chakraborty, T., and X. Lee. 2018. A simplified urban-extent algorithm to characterise surface urban heat islands on a global scale and examine vegetation control on their spatiotemporal variability. International Journal of Applied Earth Observation and Geoinformation 74: 269–80. https://doi.org/10.1016/j.jag.2018.09.015
Chase, M.J., S. Schlossberg, C.R. Griffin, et al. 2016. Continent-wide survey reveals massive decline in African savannah elephants. PeerJ 4:e2354. https://doi.org/10.7717/peerj.2354
Christy, B., and B. Stirton. 2015. How killing elephants finances terror in Africa. National Geographic. http://on.natgeo.com/1I5N2aO
Coetzee, J.A., and M.P. Hill. 2012. The role of eutrophication in the biological control of water hyacinth, Eichhornia crassipes, in South Africa. BioControl 57: 247–61. https://doi.org/10.1007/s10526-011-9426-y
Concha, C., R. Cañas, J. Macuer, et al. 2017. Disease prevention: An opportunity to expand edible plant-based vaccines? Vaccines 5: 14. https://doi.org/10.3390/vaccines5020014
Covey, R., and W.S. McGraw. 2014. Monkeys in a West African bushmeat market: Implications for Cercopithecid conservation in eastern Liberia. Tropical Conservation Science 7: 115–25. https://doi.org/10.1177/194008291400700103
Cox, T.M., R.L. Lewison, R. Zydelis, et al. 2007. Comparing effectiveness of experimental and implemented bycatch reduction measures: The ideal and the real. Conservation Biology 21: 1155–64. https://doi.org/10.1111/j.1523-1739.2007.00772.x
Craigie, I.D., J.E.M. Baillie, A. Balmford, et al. 2010. Large mammal population declines in Africa’s protected areas. Biological Conservation 143: 2221–28. https://doi.org/10.1016/j.biocon.2010.06.007
Croxall, J.P., S.H. Butchart, B. Lascelles, et al. 2012. Seabird conservation status, threats and priority actions: A global assessment. Bird Conservation International 22: 1–34. https://doi.org/10.1017/S0959270912000020
Cruise, A. 2017. Ten more elephants poisoned by poachers in Zim. Guardian. https://gu.com/p/6k6tx
Cumming, D.H.M., and G.S. Cumming. 2015. One Health: An ecological and conservation perspective. In: One Health: The Theory and Practice of Integrated Health Approaches, ed. by J. Zinsstag, et al. (Wallingford: CAB International).
Daszak, P., A.A. Cunningham, and A.D. Hyatt. 2000. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science 287: 443–49. https://doi.org/10.1126/science.287.5452.443
Davies, D., B.J. Dilley, A.L. Bond, et al. 2015. Trends and tactics of mouse predation on Tristan Albatross Diomedea dabbenena chicks at Gough Island, South Atlantic Ocean. Avian Conservation and Ecology 10: 5. http://doi.org/10.5751/ACE-00738-100105
de Graaf, M., M.A.M. Machiels, T. Wudneh, et al. 2004. Declining stocks of Lake Tana’s endemic Barbus species flock (Pisces, Cyprinidae): Natural variation or human impact? Biological Conservation 116: 277–87. http://doi.org/10.1016/S0006-3207(03)00198-8
de Greef, K. 2019. 500 vultures killed in Botswana by poachers’ poison, government says. New York Times. https://nyti.ms/2FpkfTf
Deribe, E., B.O. Rosseland, R. Borgstrøm, et al. 2011. Bioaccumulation of persistent organic pollutants (POPs) in fish species from Lake Koka, Ethiopia: The influence of lipid content and trophic position. Science of The Total Environment 410–11: 135–45. http://doi.org/10.1016/j.scitotenv.2011.09.008
Desta, Z., R. Borgstrøm, B.O. Rosseland, et al. 2006. Major difference in mercury concentrations of the African big barb, Barbus intermedius (R.) due to shifts in trophic position. Ecology of Freshwater Fish 15: 532–43. https://doi.org/10.1111/j.1600-0633.2006.00193.x
Dilley, B.J., D. Davies, A.L. Bond, et al. 2015a. Effects of mouse predation on burrowing petrel chicks at Gough Island. Antarctic Science 27: 543–53. https://doi.org/10.1017/S0954102015000279
Dilley, B.J., S. Schoombie, J. Schoombie, et al. 2015b. ‘Scalping’ of albatross fledglings by introduced mice spreads rapidly at Marion Island. Antarctic Science 28: 73–80. https://doi.org/10.1017/S0954102015000486
Dodoo, D.K., D.K. Essumang, and J.W.A. Jonathan. 2013. Accumulation profile and seasonal variations of polychlorinated biphenyls (PCBs) in bivalves Crassostrea tulipa (oysters) and Anadara senilis (mussels) at three different aquatic habitats in two seasons in Ghana. Ecotoxicology and Environmental Safety 88: 26–34. http://doi.org/10.1016/j.ecoenv.2012.10.013
Dzikiti, S., M.B. Gush, D.C. le Maitre, et al. 2016. Quantifying potential water savings from clearing invasive alien Eucalyptus camaldulensis using in situ and high resolution remote sensing data in the Berg River Catchment, Western Cape, South Africa. Forest Ecology and Management 361: 69–80. https://doi.org/10.1016/j.foreco.2015.11.009
Eknath, A.E., H.B. Bentsen, P.W. Ponzoni, et al. 2007. Genetic improvement of farmed tilapias: Composition and genetic parameters of a synthetic base population of Oreochromis niloticus for selective breeding. Aquaculture 273: 1–14. https://doi.org/10.1016/j.aquaculture.2007.09.015
Ellsworth, W.L. 2013. Injection-induced earthquakes. Science 341: 1225942. https://doi.org/10.1126/science.1225942
Eriksen M., L.C.M. Lebreton, H.S. Carson, et al. 2014. Plastic pollution in the world’s oceans: More than 5 trillion plastic pieces weighing over 250,000 tons afloat at sea. PLoS ONE 9: e111913. https://doi.org/10.1371/journal.pone.0111913
Esvelt, K.M., A.L. Smidler, F. Catteruccia, et al. 2014. Emerging technology: Concerning RNA-guided gene drives for the alteration of wild populations. eLife 3: e03401. https://doi.org/10.7554/eLife.03401.001
Fa, J.E., C.A. Peres, and J. Meeuwig. 2002. Bushmeat exploitation in tropical forests: An intercontinental comparison. Conservation Biology 16: 232–37. https://doi.org/10.1046/j.1523-1739.2002.00275.x
Fa, J.E., D. Currie, and J. Meeuwig. 2003. Bushmeat and food security in the Congo Basin: Linkages between wildlife and people’s future. Environmental Conservation 30: 71–78. https://doi.org/10.1017/S0376892903000067
Fa, J.E., J. Olivero, M.A. Farfán, et al. 2016. Differences between Pygmy and non-Pygmy hunting in Congo Basin forests. PLoS ONE 11: e0161703. https://doi.org/10.1371/journal.pone.0161703
Fa, J.E., S. Seymour, J.E.F. Dupain, et al. 2006. Getting to grips with the magnitude of exploitation: Bushmeat in the Cross-Sanaga rivers region, Nigeria and Cameroon. Biological Conservation 129: 497–510. https://doi.org/10.1016/j.biocon.2005.11.031
Falchi, F., P. Cinzano, D. Duriscoe, et al. 2016. The new world atlas of artificial night sky brightness. Science Advances 2: e1600377. https://doi.org/10.1126/sciadv.1600377
Fanou, L.A., T.A. Mobio, E.E. Creppy, et al. 2006. Survey of air pollution in Cotonou, Benin—air monitoring and biomarkers. Science of The Total Environment 358: 85–96. https://doi.org/10.1016/j.scitotenv.2005.03.025
Fentiman, A., and N. Zabbey. 2015. Environmental degradation and cultural erosion in Ogoniland: A case study of the oil spills in Bodo. Extractive Industries and Society 2: 615–24. https://doi.org/10.1016/j.exis.2015.05.008
Feyisa, G.L., K. Dons, and H. Meilby. 2014. Efficiency of parks in mitigating urban heat island effect: An example from Addis Ababa. Landscape and Urban Planning 123: 87–95. https://doi.org/10.1016/j.landurbplan.2013.12.008
Firebaugh, A., and K.J. Haynes. 2016. Experimental tests of light-pollution impacts on nocturnal insect courtship and dispersal. Oecologia 182: 1203–11. https://doi.org/10.1007/s00442-016-3723-1
Fogell, D.J., K.A. Tolley, and G.J. Measey. 2013. Mind the gaps: Investigating the cause of the current range disjunction in the Cape Platanna, Xenopus gilli (Anura: Pipidae). PeerJ 1: e166. https://doi.org/10.7717/peerj.166
Foxcroft, L.C., D.M. Richardson, and J.R.U. Wilson. 2008. Ornamental plants as invasive aliens: Problems and solutions in Kruger National Park, South Africa. Environmental Management 41: 32–51. https://doi.org/10.1007/s00267-007-9027-9
Galloway, T.S., and C.N. Lewis. 2016. Marine microplastics spell big problems for future generations. Proceedings of the National Academy of Sciences 113: 2331–33. https://doi.org/10.1073/pnas.1600715113
Geddie, J. 2019. Singapore seizes record haul of pangolin scales enroute to Vietnam. Reuters. https://reut.rs/2WMDtsh
Genton, C., R. Cristescu, S. Gatti, et al. 2012. Recovery potential of a western lowland gorilla population following a major Ebola outbreak: Results from a ten-year study. PLoS ONE 7: e37106. https://doi.org/10.1371/journal.pone.0037106
Gewin, V. 2003. Genetically modified corn—Environmental benefits and risks. PLoS Biology 1: e8. https://doi.org/10.1371/journal.pbio.0000008
Gilbert, N. 2010. GM crop escapes into the American wild. Nature News https://doi.org/10.1038/news.2010.393
Gottdenker, N.L., D.G. Streicker, C.L. Faust, et al. 2014. Anthropogenic land use change and infectious diseases: A review of the evidence. EcoHealth 11: 619632. https://doi.org/10.1007/s10393-014-0941-z
Goulson, D., E. Nicholls, C. Botías, et al. 2015. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 347: 1255957. https://doi.org/10.1126/science.1255957
Haim, A., and B.A. Portnov. 2013. Light Pollution as a New Risk Factor for Human Breast and Prostate Cancers (Dordrecht: Springer). https://doi.org/10.1007/978-94-007-6220-6
Harding, W.R. 2015. Living with eutrophication in South Africa: A review of realities and challenges. Transactions of the Royal Society of South Africa 70: 155–71. https://doi.org/10.1080/0035919X.2015.1014878
Hargrove, J.W. 2003. Tsetse eradication: Sufficiency, necessity and desirability (Edinburgh: DFID Animal Health Programme, Centre for Tropical Veterinary Medicine, University of Edinburgh). https://assets.publishing.service.gov.uk/media/57a08d1be5274a31e0001656/RLAHtsetse_Erad.pdf
Hauenstein, S., M. Kshatriya, J. Blanc, et al. 2019. African elephant poaching rates correlate with local poverty, national corruption and global ivory price. Nature Communications 10: 2242. https://doi.org/10.1038/s41467-019-09993-2
Hayes, T.B., and K.P. Menendez. 1999. The effect of sex steroids on primary and secondary sex differentiation in the sexually dichromatic reedfrog (Hyperolius argus: Hyperolidae) from the Arabuko Sokoke Forest of Kenya. General and Comparative Endocrinology 115: 188–99. https://doi.org/10.1006/gcen.1999.7321
Hirschfeld M., D.C. Blackburn, T.M. Doherty-Bone, et al. 2016. Dramatic declines of montane frogs in a central African biodiversity hotspot. PLoS ONE 11: e0155129. https://doi.org/10.1371/journal.pone.0155129
Howarth, R.W. 2014. A bridge to nowhere: Methane emissions and the greenhouse gas footprint of natural gas. Energy Science and Engineering 2: 47–60. https://doi.org/10.1002/ese3.35
Hwang, Y.-T., D.M.W. Frierson, and S.M. Kang. 2013. Anthropogenic sulfate aerosol and the southward shift of tropical precipitation in the late 20th century. Geophysical Research Letters 40: 2845–50. https://doi.org/10.1002/grl.50502
IUCN. 2019. The IUCN Red List of Threatened Species. http://www.iucnredlist.org
Karagulian, F., C.A. Belis, C.C.C. Dora, et al. 2015. Contributions to cities’ ambient particulate matter (PM): A systematic review of local source contributions at global level. Atmospheric Environment 120: 475–83. https://doi.org/10.1016/j.atmosenv.2015.08.087
Karami, A., A. Golieskardi, C.K. Choo, et al. 2017. The presence of microplastics in commercial salts from different countries. Scientific Reports 7: 46173. https://doi.org/10.1038/srep46173
Kebede, A.T., and D.L. Coppock. 2015. Livestock-mediated dispersal of Prosopis juliflora imperils grasslands and the endangered Grevy’s zebra in northeastern Ethiopia. Rangeland Ecology and Management 68: 402–07. https://doi.org/10.1016/j.rama.2015.07.002
Kern, J.M., and A.N. Radford. 2016. Anthropogenic noise disrupts use of vocal information about predation risk. Environmental Pollution 218: 988–95. http://doi.org/10.1016/j.envpol.2016.08.049
King J., J.A. Cambray, and N.D. Impson. 1998. Linked effects of dam-released floods and water temperature on spawning of the Clanwilliam yellowfish Barbus capensis. Hydrobiologia 384: 245–65. https://doi.org/10.1023/A:1003481524320
Kissui, B.M., and C. Packer. 2004. Top-down population regulation of a top predator: Lions in the Ngorogonro Crater. Proceedings of the Royal Society B 271: 1867–74. https://doi.org/10.1098/rspb.2004.2797
Knop, E., L. Zoller, R. Ryser, et al. 2017. Artificial light at night as a new threat to pollination. Nature 548: 206–09. https://doi.org/10.1038/nature23288
Koper, R.P., and S. Plön. 2012. The potential impacts of anthropogenic noise on marine animals and recommendations for research in South Africa. EWT Research and Technical Paper 1 (Johannesburg: EWT).
Kosuth, M., S.A. Mason, and C. Tyree. 2017. Synthetic Polymer Contamination in Global Drinking Water (Washington: Orb Media). https://orbmedia.org/stories/Invisibles_plastics
Kouassi, K.S., S. Billet, G. Garçon, et al. 2010. Oxidative damage induced in A549 cells by physically and chemically characterized air particulate matter (PM2. 5) collected in Abidjan, Côte d’Ivoire. Journal of Applied Toxicology 30: 310–20. https://doi.org/10.1002/jat.1496
Kunc, H.P., K.E. McLaughlin, and R. Schmidt. 2016. Aquatic noise pollution: Implications for individuals, populations, and ecosystems. Proceedings of the Royal Society B 283: 20160839. https://doi.org/10.1098/rspb.2016.0839
Landrigan, P.J., R. Fuller, N.J.R. Acosta, et al. 2018. The Lancet Commission on pollution and health. Lancet 391: P462–P512. https://doi.org/10.1016/S0140-6736(17)32345-0
le Maitre, D.C., G.G. Forsyth, S. Dzikiti, et al. 2016. Estimates of the impacts of invasive alien plants on water flows in South Africa. Water SA 42: 659–72. http://dx.doi.org/10.4314/wsa.v42i4.17
Lebreton, L.C.M., J. van der Zwet, J.-W. Damsteeg, et al. 2017. River plastic emissions to the world’s oceans. Nature Communications 8: 15611. https://doi.org/10.1038/ncomms15611
Lindsey P.A., V.R. Nyirenda, J.L. Barnes, et al. 2014. Underperformance of African protected area networks and the case for new conservation models: Insights from Zambia. PLoS ONE 9: e94109. https://doi.org/10.1371/journal.pone.0094109
Lindsey, P.A., G. Balme, M. Becker, et al. 2013. The bushmeat trade in African savannas: Impacts, drivers, and possible solutions. Biological Conservation 160: 80–96. https://doi.org/10.1016/j.biocon.2012.12.020
Lovett, B., E. Bilgo, S.A. Millogo, et al., 2019. Transgenic Metarhizium rapidly kills mosquitoes in a malaria-endemic region of Burkina Faso. Science 364: 894897. https://doi.org/10.1126/science.aaw8737
Maisels, F., S. Strindberg, S. Blake, et al. 2013. Devastating decline of forest elephants in Central Africa. PLoS ONE 8: e59469. https://doi.org/10.1371/journal.pone.0059469
Manaca, M.N., J.O. Grimalt, J. Sunyer, et al. 2011. Concentration of DDT compounds in breast milk from African women (Manhiça, Mozambique) at the early stages of domestic indoor spraying with this insecticide. Chemosphere 85: 307–14. https://doi.org/10.1016/j.chemosphere.2011.06.015
Maree, B.A., R.M. Wanless, T.P. Fairweather, et al. 2014. Significant reductions in mortality of threatened seabirds in a South African trawl fishery. Animal Conservation 17: 520–29. https://doi.org/10.1111/acv.12126
Maxwell, S.L., R.A. Fuller, T.M. Brooks, et al. 2016. The ravages of guns, nets and bulldozers. Nature 536: 143–45. https://doi.org/10.1038/536143a
Mcgowan, P. 2008. WG6 CS1: African grey parrot Psittacus erithacus case study (Cancun: NDF Workshop). https://www.cites.org/sites/default/files/ndf_material/WG6-CS1-S.pdf
McKenzie, L.M., R. Guo, R.Z. Witter, et al. 2014. Birth outcomes and maternal residential proximity to natural gas development in rural Colorado. Environmental Health Perspectives 122: 412–17. https://doi.org/10.1289/ehp.1306722
McKenzie, L.M., R.Z. Witter, L.S. Newman, et al. 2012. Human health risk assessment of air emissions from development of unconventional natural gas resources. Science of the Total Environment 424: 79–87. https://doi.org/10.1016/j.scitotenv.2012.02.018
McKinney, M.A., K. Dean, N.E. Hussey, et al. 2016. Global versus local causes and health implications of high mercury concentrations in sharks from the east coast of South Africa. Science of the Total Environment 541: 176–83. https://doi.org/10.1016/j.scitotenv.2015.09.074
McPherson, J.M., and A.C.J. Vincent. 2004. Assessing East African trade in seahorse species as a basis for conservation under international controls. Aquatic Conservation 14: 521–38. https://doi.org/10.1002/aqc.629
Merly, L., L. Lange, M. Meÿer, et al. 2019. Blood plasma levels of heavy metals and trace elements in white sharks (Carcharodon carcharias) and potential health consequences. Marine Pollution Bulletin 142: 85–92. https://doi.org/10.1016/j.marpolbul.2019.03.018
Mihaljevič, M., V. Ettler, O. Šebek, et al. 2011. Lead isotopic and metallic pollution record in tree rings from the Copperbelt mining-smelting area, Zambia. Water, Air, and Soil Pollution 216: 657–68. http://doi.org/10.1007/s11270-010-0560-4
Miller, G.T., and S. Spoolman 2011. Sustaining the Earth (Pacific Grove: Thompson Learning).
Minnaar, C., J.G. Boyles, I.A. Minnaar, et al. 2015. Stacking the odds: Light pollution may shift the balance in an ancient predator-prey arms race. Journal of Applied Ecology 52: 522–31. https://doi.org/10.1111/1365-2664.12381
Morell, M., A. Brownlow, B. McGovern, et al., 2017. Implementation of a method to visualize noise-induced hearing loss in mass stranded cetaceans. Scientific Reports 7: 41848. https://doi.org/10.1038/srep41848
Muir, W.M., and R.D. Howard. 2004. Characterization of environmental risk of genetically engineered (GE) organisms and their potential to control exotic invasive species. Aquatic Sciences 66: 414–20. https://doi.org/10.1007/s00027-004-0721-x
Nasi, R., A. Taber, and N. van Vliet. 2011. Empty forests, empty stomachs? Bushmeat and livelihoods in the Congo and Amazon Basins. International Forestry Review 13: 355–68. http://doi.org/10.1505/146554811798293872
Nyenje, P.M., J.W. Foppen, S. Uhlenbrook, et al. 2010. Eutrophication and nutrient release in urban areas of sub-Saharan Africa—a review. Science of The Total Environment 408: 447–55. https://doi.org/10.1016/j.scitotenv.2009.10.020
O’Hanlon, S.J., A. Rieux, R.A. Farrer, et al. 2018. Recent Asian origin of chytrid fungi causing global amphibian declines. Science 360: 621–27. https://doi.org/10.1126/science.aar1965
Osborn, S.G., A. Vengosh, N.R. Warner, et al. 2011. Methane contamination of drinking water accompanying gas-well drilling and hydraulic fracturing. Proceedings of the National Academy of Sciences 108: 8172–76. https://doi.org/10.1073/pnas.1100682108
Ouyang, J.Q., M. de Jong, R.H.A. van Grunsven, et al. 2017. Restless roosts: Light pollution affects behavior, sleep, and physiology in a free‐living songbird. Global Change Biology 23: 4987–94. https://doi.org/10.1111/gcb.13756
Parks and Wildlife Service 2014. Evaluation report August 2014: Macquarie Island pest eradication project (Hobart: DPIPWE). https://www.parks.tas.gov.au/file.aspx?id=31160
Pauly, D., D. Belhabib, R., Blomeyer, et al., 2014. China’s distant-water fisheries in the 21st century. Fish and Fisheries 15: 474–88. https://doi.org/10.1111/faf.12032
Petkova, E.P., D.W. Jack, N.H. Volavka-Close, et al. 2013. Particulate matter pollution in African cities. Air Quality, Atmosphere and Health 6: 603–14. https://doi.org/10.1007/s11869-013-0199-6
Pettis, J.S., E.M. Lichtenberg, M. Andree, et al. 2013. Crop pollination exposes honey bees to pesticides which alters their susceptibility to the gut pathogen Nosema ceranae. PloS ONE 8.7: e70182. https://doi.org/10.1371/journal.pone.0070182
Piaggio, A.J., G. Segelbacher, P.J. Seddon, et al. 2017. Is it time for synthetic biodiversity conservation? Trends in Ecology and Evolution 32: 97–107. https://doi.org/10.1016/j.tree.2016.10.016
Pirk, C.W.W., U. Strauss, A.A. Yusuf, et al. 2015. Honeybee health in Africa—a review. Apidologie 1–25. https://doi.org/10.1007/s13592-015-0406-6
Poulsen, J.R., C.J. Clark, G. Mavah, et al. 2009. Bushmeat supply and consumption in a tropical logging concession in northern Congo. Conservation Biology 23: 1597–608. https://doi.org/10.1111/j.1523-1739.2009.01251.x
Pringle, R.M. 2005. The origins of the Nile perch in Lake Victoria. BioScience 55: 780–87. https://doi.org/10.1641/0006-3568(2005)055[0780:TOOTNP]2.0.CO;2
Prüss-Ustün, A., J. Wolf, C. Corvalán, et al. 2016. Preventing disease through healthy environments: A global assessment of the burden of disease from environmental risks (Geneva: WHO). http://apps.who.int/iris/bitstream/10665/204585/1/9789241565196_eng.pdf
Quédraogo, O., and M. Amyot. 2013. Mercury, arsenic and selenium concentrations in water and fish from sub-Saharan semi-arid freshwater reservoirs (Burkina Faso). Science of The Total Environment 444: 243–54. https://doi.org/10.1016/j.scitotenv.2012.11.095
Rabinowitz, P.M., I.B. Slizovskiy, V. Lamers, et al. 2015. Proximity to natural gas wells and reported health status: Results of a household survey in Washington County, Pennsylvania. Environmental Health Perspectives 123: 21–26. https://doi.org/10.1289/ehp.1307732
Ramos, R., and D. Grémillet. 2013. Overfishing in West Africa by EU vessels. Nature 496: 300. https://doi.org/10.1038/496300a
Robinson, B.H. 2009. E-waste: An assessment of global production and environmental impacts. Science of The Total Environment 408: 183–91. https://doi.org/10.1016/j.scitotenv.2009.09.044
Roca, A.L., Y. Ishida, A.L. Brandt, et al. 2015. Elephant natural history; a genomic perspective. Annual Review of Animal BioScience 3: 139–67. https://doi.org/10.1146/annurev-animal-022114-110838
Roche, H., and A. Tidou. 2009. First ecotoxicological assessment assay in a hydroelectric reservoir: The Lake Taabo (Côte d’Ivoire). Bulletin of Environmental Contamination and Toxicology 82: 322–26. https://doi.org/10.1007/s00128-008-9572-9
Roelf, W. 2016. South Africa to start shale gas exploration in next year. Reuters. http://reut.rs/1U1RNZQ
Rogan, M.S., P.A. Lindsey, C.J. Tambling, et al. 2017. Illegal bushmeat hunters compete with predators and threaten wild herbivore populations in a global tourism hotspot. Biological Conservation 210: 233–42. https://doi.org/10.1016/j.biocon.2017.04.020
Rosin, C., and J.R. Poulsen. 2016. Hunting-induced defaunation drives increased seed predation and decreased seedling establishment of commercially important tree species in an Afrotropical forest. Forest Ecology and Management 382: 206–13. https://doi.org/10.1016/j.foreco.2016.10.016
Ross, P.S., and L.S. Birnbaum. 2003. Integrated human and ecological risk assessment: A case study of persistent organic pollutants (POPs) in humans and wildlife. Human and Ecological Risk Assessment 9: 303–24. https://doi.org/10.1080/727073292
Rybnikova, N.A., A. Haim, and B.A. Portnov. 2016. Does artificial light-at-night exposure contribute to the worldwide obesity pandemic? International Journal of Obesity 40: 815–23. https://doi.org/10.1038/ijo.2015.255
Schmidt, C., T. Krauth, and S. Wagner. 2017. Export of plastic debris by rivers into the sea. Environmental Science and Technology 51: 12246–53. https://doi.org/10.1021/acs.est.7b02368
Schneeberger, K., and C.C. Voigt. 2016. Zoonotic viruses and conservation of bats. In: Bats in the Anthropocene: Conservation of Bats in a Changing World, ed. by C.C. Voigt and T. Kingston (Cham: Springer). https://doi.org/10.1007/978-3-319-25220-9
Schreuder, E., and S. Clusella-Trullas. 2016. Exotic trees modify the thermal landscape and food resources for lizard communities. Oecologia 182: 1213–25. https://doi.org/10.1007/s00442-016-3726-y
Shannon, G., M.F. McKenna, L.M. Angeloni, et al. 2015. A synthesis of two decades of research documenting the effects of noise on wildlife. Biological Reviews 91: 982–1005. https://doi.org/10.1111/brv.12207
Sitoki, L., R. Kurmayer, and E. Rott. 2012. Spatial variation of phytoplankton composition, biovolume, and resulting microcystin concentrations in the Nyanza Gulf (Lake Victoria, Kenya). Hydrobiologia 691: 109–22. https://doi.org/10.1007/s10750-012-1062-8
Spinage, C.A. 1973. A review of ivory exploitation trends in Africa. East African Wildlife Journal 11: 281–89. https://doi.org/10.1111/j.1365-2028.1973.tb00093.x
Stauffer, J.R., H. Madsen, K. McKaye, et al. 2006. Schistosomiasis in Lake Malawi: Relationship of fish and intermediate host density to prevalence of human infection. EcoHealth 3: 22–27. https://doi.org/10.1007/s10393-005-0007-3
Strauss, U, V. Dietemann, H. Human, et al. 2016. Resistance rather than tolerance explains survival of savannah honeybees (Apis mellifera scutellata) to infestation by the parasitic mite, Varroa destructor. Parasitology 143: 374–87. https://doi.org/10.1017/S0031182015001754
Street, R.A. 2012. Heavy metals in medicinal plant products—an African perspective. South African Journal of Botany 82: 67–74. https://doi.org/10.1016/j.sajb.2012.07.013
Sussarellu, R., M. Suquet, Y. Thomas, et al. 2016. Oyster reproduction is affected by exposure to polystyrene microplastics. Proceedings of the National Academy of Sciences 113: 2430–35. https://doi.org/10.1073/pnas.1519019113
Tarrant, J, D. Cilliers, L.H. du Preez, et al. 2013. Spatial assessment of amphibian chytrid fungus (Batrachochytrium dendrobatidis) in South Africa confirms endemic and widespread Infection. PLoS ONE 8: e69591. https://doi.org/10.1371/journal.pone.0069591
Thibault, M., and S. Blaney. 2003. The oil industry as an underlying factor in the bushmeat crisis in Central Africa. Conservation Biology 17: 1807–13. https://doi.org/10.1111/j.1523-1739.2003.00159.x
Tuakuila, J. 2013. S-phenylmercapturic acid (S-PMA) levels in urine as an indicator of exposure to benzene in the Kinshasa population. International Journal of Hygiene and Environmental Health 216: 494–98. https://doi.org/10.1016/j.ijheh.2013.03.012
UNDP. 2006. Niger Delta human development report (Abuja: UNDP). http://hdr.undp.org/sites/default/files/nigeria_hdr_report.pdf
UNEP-WCMC. 2007. Wildlife Trade 2005: An analysis of the European community and candidate countries’ annual reports to CITES (Cambridge: UNEP-WCMC). http://ec.europa.eu/environment/cites/pdf/2007_yearbook.pdf
UNEP-WCMC. 2008. Monitoring of international trade in ornamental fish (Cambridge: UNEP-WCMC). http://ec.europa.eu/environment/cites/pdf/reports/ornamental_fish.pdf
UNEP. 2006. Report on atmosphere and air pollution. In: African Regional Implementation Review for the 14th Session of the Commission on Sustainable Development (New York: UNEP). https://sustainabledevelopment.un.org/content/documents/ecaRIM_bp2.pdf
Uyi, O.O., F. Ekhator, C.E. Ikuenobe, et al. 2014. Chromolaena odorata invasion in Nigeria: A case for coordinated biological control. Management of Biological Invasions 5: 377–93. http://doi.org/10.3391/mbi.2014.5.4.09
van der Merwe, P., Saayman, M., and Rossouw, R. 2015. The economic impact of hunting in the Limpopo Province. Journal of Economic and Financial Sciences 8: 223–42. https://hdl.handle.net/10520/EJC170564
Vanthomme, H., B. Bellé, and P.M. Forget. 2010. Bushmeat hunting alters recruitment of large‐seeded plant species in Central Africa. Biotropica 42: 672–79. https://doi.org/10.1111/j.1744-7429.2010.00630.x
Villamagna, A.M., and B.R. Murphy. 2010. Ecological and socio-economic impacts of invasive water hyacinth (Eichhornia crassipes): A review. Freshwater Biology 55: 282–98. https://doi.org/10.1111/j.1365-2427.2009.02294.x
Wanless, R.M., and B.A. Maree. 2014. Problems and solutions for seabird bycatch in trawl fisheries. Animal Conservation 17: 534. https://doi.org/10.1111/acv.12183
Waters, C.N., J. Zalasiewicz, C. Summerhayes, et al. 2016. The Anthropocene is functionally and stratigraphically distinct from the Holocene. Science 351: aad2622. https://doi.org/10.1126/science.aad2622
Watkins, B.P., S.L. Petersen, and P.G. Ryan. 2008. Interaction between seabirds and deep-water hake trawl gear: An assessment of impacts in South African waters. Animal Conservation 11: 247–54. http://doi.org/10.1111/j.1469-1795.2008.00192.x
Weldon, C, L.H. Du Preez, A.D. Hyatt, et al. 2004. Origin of the amphibian chytrid fungus. Emerging Infectious Diseases 10: 2100–05. https://doi.org/10.3201/eid1012.030804
WHO (World Health Organisation). 2013. Review of evidence on health aspects of air pollution—REVIHAAP project (Bonn: WHO). http://www.euro.who.int/__data/assets/pdf_file/0004/193108/REVIHAAP-Final-technical-report.pdf
Wilcox, C., E. van Sebille, and B.D. Hardesty. 2015. Threat of plastic pollution to seabirds is global, pervasive, and increasing. Proceedings of the National Academy of Sciences 112: 11899–904. https://doi.org/10.1073/pnas.1502108112
Williams V.L., A.B. Cunningham, A.C. Kemp, et al. 2014. Risks to birds traded for African traditional medicine: A quantitative assessment. PLoS ONE 9: e105397. https://doi.org/10.1371/journal.pone.0105397
Williams, T.M., T.L. Kendall, B.P. Richter, et al. 2017. Swimming and diving energetics in dolphins: A stroke-by-stroke analysis for predicting the cost of flight responses in wild odontocetes. Journal of Experimental Biology 220: 1135–45. https://doi.org/10.1242/jeb.154245
Wittemyer, G., J.M. Northrup, J. Blanc, et al. 2014. Illegal killing for ivory drives global decline in African elephants. Proceedings of the National Academy of Sciences 111: 13117–21. https://doi.org/10.1073/pnas.1403984111
Wolfaardt, A.C., A.J. Williams, L.G. Underhill, et al. 2009. Review of the rescue, rehabilitation and restoration of oiled seabirds in South Africa, especially African penguins Spheniscus demersus and Cape gannets Morus capensis, 1983–2005. African Journal of Marine Science 31: 31–54. https://doi.org/10.2989/AJMS.2009.31.1.3.774
Wood, K.L., B. Tenger, N.V. Morf, et al. 2014. Report to CITES: CITES-listed species at risk from the illegal trafficking of bushmeat; Results of a 2012 study in Switzerland’s international airports (Bonn CITES). https://doi.org/10.5167/uzh-111850
Worm, B., E.B. Barbier, N. Beaumont, et al. 2006. Impacts of biodiversity loss on ocean ecosystem services. Science 314: 787–90. https://doi.org/10.1126/science.1132294
Zunckel, M., K. Venjonoka, J.J. Pienaar, et al. 2004. Surface ozone over southern Africa: Synthesis of monitoring results during the Cross-border Air Pollution Impact Assessment Project. Atmospheric Environment 38: 6139–47. http://doi.org/10.1016/j.atmosenv.2004.07.029

