7.3: Glycoconjugates - Proteoglycans, Glycoproteins, Glycolipids and Cell Walls
- Page ID
- 14957
Many proteins, especially those destined for secretion or insertion into membranes, are post-translationally modified by the attachment of carbohydrates. They are usually attached through either Asn or Ser side chains. Carbohydrate modifications on the protein appear to be involved in the recognition of other binding molecules, prevention of aggregation during protein folding, protection from proteolysis, and increases the half-life of the proteins. In contrast to a protein sequence determined by a DNA template, sugars are attached to proteins by enzymes that recognize appropriate sites on proteins and attach the sugars. Since there are many sugars with many functional groups that can serve as potential attachment sites, the structures of the oligosaccharides attached to proteins are enormously varied, complex, and hence "information rich" compared to linear or folded polymers like DNA and proteins.
N-linked Glycoproteins
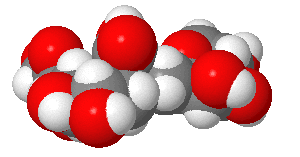
These contain carbohydrates attached through either a GlcNAc or GalNAc to an Asn in a X-Asn-X-Thr sequence of the protein. There are three types of N-linked glycoproteins, high mannose, complex, and hybrid. They all contain the same core oligosaccharide - (Man)3(GlcNAc)2 attached to Asn as shown in Figure \(\PageIndex{1}\).
Table \(\PageIndex{1}\) below shows the SNFG representation for the main core and variant glycans in N-linked glycoproteins. Note that the designation of α2 implies an α(1→2) linkage. Unless otherwise stated the linkage is presumed to start from carbon 1.
| Core | |
| High mannose | |
| Complex | |
| Mixed hybrid |
Table \(\PageIndex{1}\): SNFG representation for the main core and variant glycans in N-linked glycoproteins
Complex N-linked glycans don't contain mannose outside of the core glycan and have GlcNAc attached to the branching mannoses in the core structure. The complex glycan shown above has a Gal(β1,4)GlcNAc sequence which could be named as the disaccharide lactosamine. Often, lactosamine repeats in the sequence.
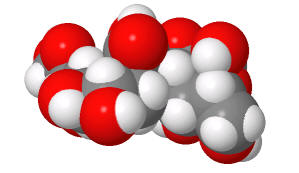
Hybrid glycans have both unsubstituted terminal mannoses (as in the high-mannose type) and substituted mannoses with an N-acetylglucosamine attached (as in the complex type. GlcNAc residues added to the core in the hybrid and complex N-glycoproteins are called antennae. Figure \(\PageIndex{2}\) shows an example of a biantennary N-linked glycan with two GlcNAc branches linked to the core. The core is outlined in red and the two GlcNAcs are labeled 1 and 2.
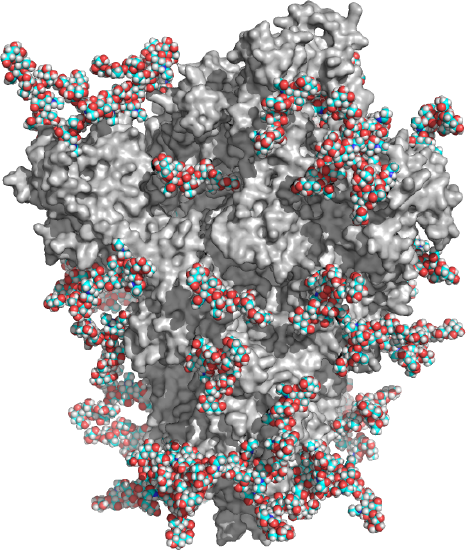
Complex glycans also have bi-, tri- and tetraantennary forms and comprise most of N-glycans. As shown in Table \(\PageIndex{1}\) above, complex N-linked glycans usually end with sialic acid residues. About 50% of the surface area of the COVID Sars-2 spike protein is covered with glycans as shown in the model structure in Figure \(\PageIndex{3}\). The protein surface is gray and the glycans (biantennary LacNAc N-glycans) in spacefill CPK with carbon in cyan.
In the hybrid oligosaccharide shown above, one terminus contains Gal(β1,4)GlcNAc. However, in all other mammals except man, apes, and old-world monkeys, an additional Gal is often connected in an α1,3 link to the Gal to give a terminus of Gal(α1,3)Gal(β1,4)GlcNAc. These animals have an additional enzyme, an α1,3 Gal transferase. Bacteria also have this enzyme, and since we have been exposed to this link through bacterial infection, we mount an immune response against it. Why is this important? Pig hearts turn out to be similar to human hearts, so they might be good candidates for transplantation into humans (xenotransplants). However, the Gal-α1,3-Gal link is recognized as foreign, and we mount a significant immune response against it. Several biotech firms are trying to delete the pig α1,3 Gal transferase which would prevent the addition of the terminal Gal, and make them good donors for transplanted hearts.
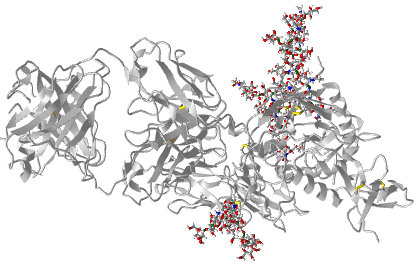
Figure \(\PageIndex{4}\) shows an interactive iCn3D model of an N-linked glycoprotein, human beta-2-glycoprotein-I (Apolipoprotein-H) (1C1Z).
_(1C1Z).png?revision=1&size=bestfit&width=443&height=226)
The glycan structures for the beta-2-glycoprotein-I are shown below. Identify the monosaccharides in each and specific to which asparagine they are linked.
- Answer
-
add answer here.
Figure \(\PageIndex{5}\) shows an interactive iCn3D model of the GP120 HIV protein that contains a high mannose, complex and hybrid N-linked glycans. Most glycoproteins in the Protein Data Bank do not contain attached glycans. The glycans here were added with the program GlyProt at 3 of 17 possible Asn residues that would presumably have attached glycans. Use your mouse or key paid to hover over the monomers in the attached glycans. Abbreviations for the given residues in the model are as follows: adm = alpha-D-Man, bdg= beta-D-Glc or Gal, adn = alpha-D-neuramindase.
Which Asn chains contain the high Man, complex, and hybrid glycans?
- Answer
-
Add answer here
The coronavirus pandemic of 2020-23 has been deadly (over 1.1 million deaths in the USA alone as of February 8, 2023 and 6.8 million around the world). However, the 1918 influenza pandemic was far worse with an estimated 650,000 deaths in the USA and 50 million around the world in a population since the population was less than 1/3 of the present. However, the number of USA deaths during the Covid-19 pandemic is greater than during the 1918 Flu pandemic. An additional 3 million deaths in the USA were probably prevented by vaccination as of December 2022. A large number of deaths in the developing world would have been prevented if wealthy nations allocated more resources to produce and distribute the vaccines. The evolution of the virus in nonvaccinated areas might come back to haunt wealthy countries if present vaccines become ineffective against the mutants. A worse pandemic might await us. An avian version of the influenza virus (H5N1), presently endemic in wild birds and now found in mink populations, has infected 240 people as of January 2023 and killed 56% of them. A quick note: the 1918 pandemic affected youth the most.
The influenza virus, a simple yet deadly virus, is shown below in Figure \(\PageIndex{6}\). It interacts with human cells through a surface protein, hemagglutinin (HA).
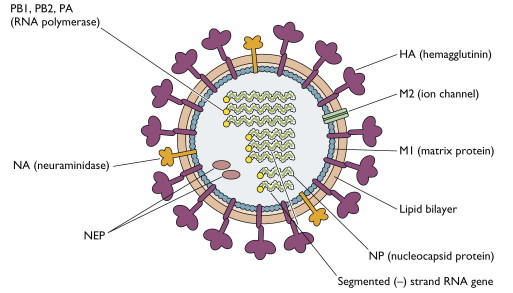
The virus binds to host cells through the interaction of HA with cell surface carbohydrates. Once bound, the virus internalizes, ultimately leading to the release of the RNA genome of the virus into the host cell.
The hemagglutinin protein is the most abundant protein on the viral surface. 15 avian and mammalian variants have been identified (based on antibody studies). Only 3 adapted to humans in the last 100 yr, giving pandemic strains H1 (1918), H2 (957) and H3 (1968). Three recent avian variants (H5, H7, and H9) jump directly to humans recently but have low human-to-human transmissibility.
The influenza hemagglutinin protein has the following characteristics:
- the mature form is a homotrimer (3 identical protein subunits), MW 220,000 with multiple sites for covalent attachment of sugars. Hemagglutinin is a glycoprotein.
- each monomer is synthesized as a single polypeptide chain precursor (HA0), which is cleaved into HA1 and HA2 subunits by the protease trypsin in epithelial cells of the lung.
- structure known for human (H3), swine (H9), avian (H5) subtypes.
Hemagglutinin binds to sialic acid (Sia) covalently attached to many cell membrane glycoproteins. The sialic acid is usually connected through an α(2,3) or α(2,6) link to galactose on N-linked glycoproteins. The subtypes found in avian (and equine) influenza isolates bind preferentially to Sia (α2,3) Gal which predominates in the avian GI tract where viruses replicate. Human influenza isolates prefer Sia α(2,6)
Sia (α2,3) Gal which predominates in the avian GI tract where viruses replicate. Human influenza isolates prefer Sia α(2,6) Gal. The human virus of H1, H2, and H3 subtypes (causes of the 1918, 1957, and 1968 pandemics) recognize Sia α(2,6) Gal, the major form in the human respiratory tract. The swine influenza HA binds to Sia α(2,6) Gal and some Sia (α2,3) Gal both of which are found in swine. The structures of the Sia-Gal disaccharide are shown in Table \(\PageIndex{2}\) below.
| Sia α(2,6) Gal (Human) | Sia α(2,3) Gal (Avian and some Swine) |
|
|
|
|
(made with Sweet, with an OH, not AcNH on sialic acid on C5) |
(made with Sweet, with an OH, not AcNH on sialic acid on C5) |
Table \(\PageIndex{2}\): Structures of Sia α(2,6) Gal (human) and Sia α(2,3) Gal (avian/swine)
The H5N1 avian flu H5N1) virus is deadly but presently lacks human-to-human transmissibility. Why? One reason is that it appears to bind deep in the lungs and is not released easily on coughing or sneezing. It appears that cell surface glycoproteins deeper in the respiratory tract have Sia (α2,3) Gal which accounts for this pathology.
Before it leaves the cell, the virus forms a bud on the intracellular side of the cell with the HA and NA in the cell membrane of the host cell. The virus in this state would not leave the cell since its HA molecules would interact with sialic acid residues in the host cell membrane, holding the virus in the membrane. Neuraminidase hydrolyzes sialic acid from cell surface glycoproteins, allowing the virus to complete the budding process and be released from the cell as new viruses. The drugs Oseltamivir (Tamiflu) and zanamivir (Relenza) bind to and inhibit neuraminidase, whose activity is necessary for viral release from infected cells. Tamiflu appears to work against N1 of the present H5N1 avian influenza viruses. Governments across the world are hopefully stockpiling this drug in case of a pandemic caused by the avian virus jumping directly to humans and becoming transmissible from human to human.
O-linked Glycoproteins
The CHOs are usually attached from a Gal (β 1,3) GalNAc to a Ser or Thr of a protein as shown in Figure \(\PageIndex{7}\).
Figure: O-linked Glycoproteins
The blood group antigens (CHOs on cells attached to either proteins or lipids) are examples. The sugars shown as chairs (in contrast to structures found in many texts) in Figure \(\PageIndex{8}\) are the blood group antigens. They are attached to a core heterosaccharide (shown as a red ellipse below), which is connected to either a membrane glycoprotein or glycolipid.
Figure \(\PageIndex{9}\) shows the SNFG representation for the A antigen in the glycolipid form.
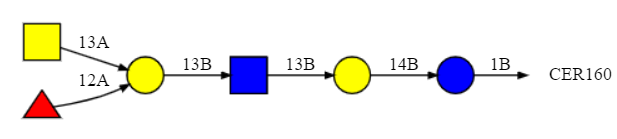
The trimeric branched residues on the left-hand side represent the A antigen shown above. The red triangle is L-fucose. Yellow represents galactose or GalNac, while blue is glucose or GlcNAc.
Proteoglycans
Some proteins are so modified with CHOs that they contain more CHOs than amino acids. Proteins linked to glycosaminoglycans are together called proteoglycans (PGs). The consists of a core protein linked to one or more glycosaminoglycans. GAGs are linear sulfated glycans which we described earlier. The structures of a few proteoglycans are known. The GAGs are O-linked to the protein, typically to a Ser of a Ser-Gly dipeptide often repeated in the protein. Some of the proteoglycans also contained N-linked oligosaccharide groups. Figure \(\PageIndex{10}\) shows a representation of proteoglycan structure.
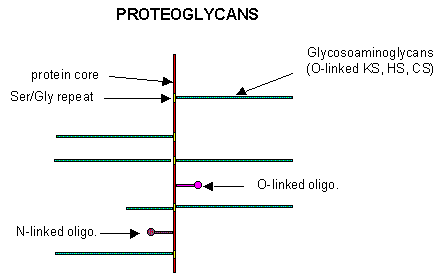
PGs can be soluble and are found in the extracellular matrix, or as integral membrane proteins. There are about 43 genes for proteoglycans. Differential splicing of the RNA transcripts give rise to soluble and transmembrane forms. Given the diversity of sugars and the varying extent of sulfation, the CHO part of PGs provides an incredible variety of binding structures at or near the cell surface. Figure \(\PageIndex{11}\) shows the variety of proteoglycans found in mammalian cells. PGs help form the extracellular matrix, which provides a rich binding environment between cells.
One PG, syndecan, binds through its intracellular domain to the internal cytoskeleton of the cell, while interacting with another protein - fibronectin - in the extracellular matrix. Fibronectin also binds other molecules which can regulate cellular growth and other interactions. PGs act like glue in connecting the extracellular and intracellular functions of the cell. There are four different core syndecan proteins (SDCs 1–4), with SDC4 lacking the cytoplasmic and transmembranes and thus is a soluble form in the intracellular matrix. The glycan components of syndecans are mostly heparan sulfate while SDC 1 and 3 also have two chondroitin sulfate chains.
Most proteins bind PGs through a PG binding motif of BBXB or BBBXXB where B is a basic amino acid. Some proteins bind to specific sequences in specific GAGs. For instance, antithrombin 3, an inhibitor of blood clotting, binds specifically to heparin. This enhances its interaction with clotting proteins such as thrombin and Factor Xa. Figure \(\PageIndex{12}\) shows an interactive iCn3D model of a 5 residue fragment of heparin interacting with the key amino acids side chains of Factor Xa (2gd4).
.png?revision=1&size=bestfit&width=275&height=201)
For those more chemically oriented, the extracellular matrix (ECM) might appear to be a nondescript mess, since chemists are used to well-defined structures. Figure \(\PageIndex{13}\) shows a cartoon of the ECM and may clarify the components. Few structure files exist for them given the inherent flexibility of the glycan components.
Cell Walls and Glycolipids
In contrast to eukaryotic cells, bacteria and plant cells have a cell wall in addition to a lipid bilayer membrane. These are essentially carbohydrate polymers that determine cell shape, affording protection from exterior pathogens, hypotonic conditions and high internal osmotic pressures, preventing swelling and bursting of the cells. This is especially important in plants, which need strength and rigidity against the "turgor" pressure of the aqueous cytoplasm against the cell membrane. This prevents wilting in plants. The cell wall in plants and probably bacteria are involved in cell signaling across the cell membrane.
Bacteria Cell Walls
Two types of cell walls occur in Nature.
a. Gram positive bacteria-
These bacteria can be stained with Gram stain. The wall consists of a GlcNAc (β 1,4) MurNAc repeat. (GlcNAc is often abbreviated as NAG while MurNAc is abbreviated as NAM.) This is similar to the GlcNAc (β 1,4) GlcNAc homopolymer chitin, except that every other GlcNAc contains a lactate molecule covalently attached in an ether-linkage to the C3 hydroxyl to form the monomer N-Acetylmuramic acid. A pentapeptide (Ala-D-isoGlu-Lys-D-Ala-D-Ala) is attached through an amide link to the carboxyl group of the lactate in MurNAc. The GlcNAc (β 1,4) MurNAc strands are covalently connected by a pentaglycine bridge through the epsilon amino group of the pentapeptide Lys on one strand and the terminal D-Ala of a pentapeptide on another strand. A small part of the structure of a gram-positive bacterial cell wall is shown in Figure \(\PageIndex{14}\). It shows one repeating GlcNAc-MurNAc disaccharide unit in front (darker) and one in the back (lighter) connected through the peptides shown.
The SNFG representation of a larger section of the gram-positive cell well is shown in Figure \(\PageIndex{15}\).
One final structure is found in Gram + peptidoglycan cell walls. Techioic acids are often attached to the carbon 6 of MurNAc. Teichoic acid is a polymer of glycerol or ribitol to which alternative GlcNAc and D-Ala are linked to the middle C of the glycerol. Multiple glycerols are linked through phosphodiester bonds. These teichoic acids often make up 50% of the dry weight of the cell wall and present a foreign (or antigenic) surface to infected hosts. These often serve as receptors for viruses that infect bacteria (called bacteriophages). Its structure is illustrated in Figure \(\PageIndex{16}\).
Notice that all monomeric units of peptidoglycan and attached teichoic acid derivatives are covalently attached on form one large molecule comprising the entire cell wall! This structure, along with the Gram-negative cell wall structures, is the largest single macromolecule in nature.
b. Gram-negative bacteria
These bacteria can NOT be stained with Gram stain. The wall consists of the same structure as in Gram-positive bacteria but the GlcNAc (β 1,4) MurNAc strands are covalently connected through a direct amide bond between a derivative of Lys, meso-diaminopimelic acid (m-A2pm), on one peptide strand and to the last D-Ala of a pentapeptide on another strand. (i.e. there is no pentaGly spacer). The connector peptide is Ala-D-isoGlu-m-A2pm-D-Ala-D-Ala
m-A2pm replaces Lys 3 of the peptide in most Gram-negative species and in Gram-positive bacteria of the genus Bacillus and mycobacteria. The stereochemistries at each chiral center are different (R and S), but because the molecule has a plane of symmetry, it is an example of a meso-compound, a diastereoisomer of a molecule, which does not have a different enantiomeric version. The structure is shown in Figure \(\PageIndex{17}\).
A small part of the structure of a Gram-negative bacterial cell wall is shown in Figure \(\PageIndex{18}\).
Figure \(\PageIndex{19}\) shows an interactive iCn3D model (actual computed model, not a crystal or NMR structure) of the Gram-negative peptidoglycan of E. Coli. The PDB coordinates were kindly provided by Jame Gumbart. The peptide part of the peptidoglycan is represented in spacefill. The repeating (GlcNac-MurNac)n and pentapeptide
In addition, Gram-negative bacterial don't have teichoic acid polymers. Rather they have a second, outer lipid bilayer. The cell wall peptidoglycan (PG) is sandwiched between the inner and outer bilayers. The space between the lipid bilayers is called the periplasm. The outer membrane is coated with a lipopolysaccharide (LPS) of varying composition. The LPS determines the antigenicity of the bacteria. The different LPS are called the O-antigens. Figure \(\PageIndex{20}\) shows the structure of the Gram-negative bacterial membrane organization. (PS is LPS, PG is peptidoglycan)

A detailed view of the structure of the lipopolysaccharide (LPS) from Salmonella tryphimurium is shown in Figure \(\PageIndex{21}\) below.
questions on this:diff btw g+ and g- cells
https://microbiologyinfo.com/differe...tive-bacteria/.
- Answer
-
Add texts here. Do not delete this text first.
c. Archaeal Cell Membranes and Walls
We have already discussed that the lipids in Archaeal cell membranes contain L (instead of D) glycerol derivatives and that ether links (more stable in reactive environments) replace ester links with isoprenoid (sometimes branched) chains replacing fatty acid chains. The cell wall is quite different as well, and some don't have one. The type of cell wall is depending on the environmental need for stability. They don't contain peptidoglycans. Figure \(\PageIndex{22}\) shows four different types.
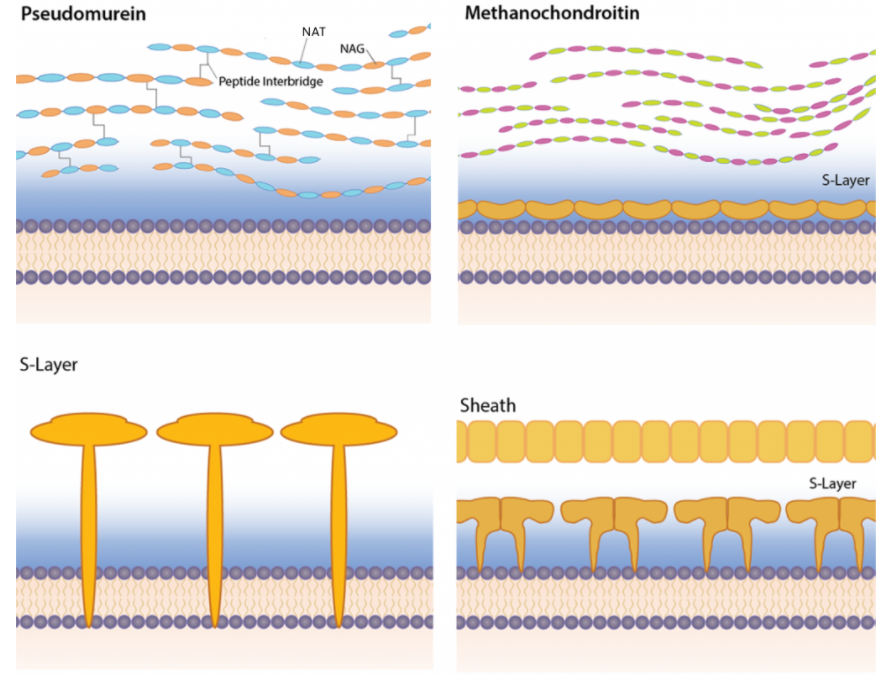
Some differences include the presence of
- pseudomurein - This is the closest to the peptidoglycans presented above. Instead of repeating disaccharide units of (NAM-NAG)n, they have a repeating disaccharide unit of N-acetylalosaminuronic acid (NAT)-NAG. The structure of NAT is shown in Figure \(\PageIndex{23}\).
Figure \(\PageIndex{23}\): N-acetylalosaminuronic acid (NAT)compared to N-acetylmuramic acid (NAM)
- methanochondroitin - This is similar to the glycosaminoglycan chondrotin sulfate
- S-Layer
- Sheath/S-Layer
d. Plant Cell Wall
If you thought bacterial cell walls were complicated, wait until you see plant cell walls! There are about 35 different types of plant cells, and each may have a different cell wall depending on the local needs of a given cell. Cells synthesize thin cell wall that extends and stay thin as the cell grows.
Figure \(\PageIndex{24}\) shows the primary cell wall of plants. The primary cell wall contains cellulose microfibrils (no surprise) and two other polymers, pectin and hemicellulose. The middle lamella consisting of pectins is somewhat analogous to the extracellular matrix discussed above.
After cell growth, the cell often synthesizes a secondary cell wall thicker than the first for extra rigidity. The enzymatic machinery for its synthesis is in the cytoplasm and the cell membrane. It is deposited between the cell membrane and the primary cell wall, as shown in the animated image shown in Figure \(\PageIndex{25}\).
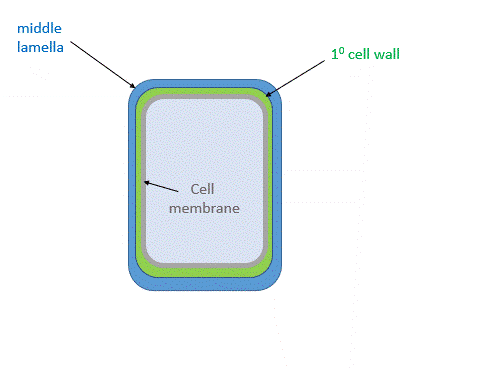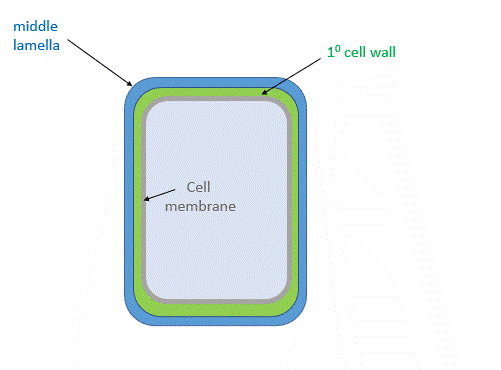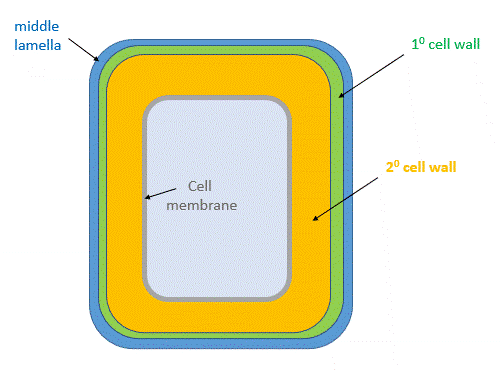
Figure \(\PageIndex{26}\) shows a structural representation of both the primary and secondary cell wall.
The middle lamella, which contains pectins, lignins and some proteins, helps "glue together" the primary cell walls of surrounding plants.
Primary Cell Wall:
The main component of the primary plant wall is the homopolymer cellulose (40% -60% mass) in which the glucose monomers are linked β(1→4)-linked into strands that collect into microfibrils through hydrogen bond interactions. Two other groups of polymers, hemicellulose and pectin make up the plant cell wall.
Hemicellulose can make up to 20-40% by the mass These polymers have β(1,4) backbones of glucose, mannose, or xylose (called xyloglucans, xylans, mannans, galactomannans, glucomannans, and galactoglucomanannans along with some β(1,3 and 1,4)-glucans. The most abundant hemicellulose in higher plants are the xyloglucans which have a cellulose backbone linked at O6 to α-D-xylose. Pectin consists of linked galacturonic acids forming homogalacturonans, rhamnogalacturonans, and rhamnogalacturonans II (RGII) [12] [13]. Homogalacturonans (α1→4) linked D-GalA making up more than 50% of the pectin
Figure \(\PageIndex{27}\) shows some variants of the cell wall components of a plant.
Secondary Cell Wall
The structure of the secondary cell wall depends on the function and environment of the cell. It contains cellulose fibers, hemicellulose and in addition a new polymer, lignin. It is abundant in xylem vessels and fiber cells of woody plants. It gives the plant extra stability and new functions, including the transport of fluids within the plant through channels.
Lignins, which can make up to 25% of the biomass weight, are made from derivatives of phenylalanine, but more directly from cinnamic acid. This derives from is made from phenylalanine which is hydroxylated and converted through other steps to hydroxycinnamyl alcohols called monolignols as shown in Figure \(\PageIndex{28}\). Three common monomer (M) derivatives, p-coumaryl, coniferyl, and sinapyl alcohols can polymerize into lignins, with the units in the polymer (P) names hydroxyphenyl, guaiacyl and syringly, respectively.
Lignols are activated phenolic compounds, which form phenoxide free radicals (catalyzed by enzymes called peroxidases), which can attack other lignols to form covalent dimers. Reaction mechanisms for the dimerization of the MS sinapyl alcohol free radical are shown as an example in Figure \(\PageIndex{29}\).
Figure \(\PageIndex{29}\): Dimers of lignols
Now imagine this polymerization continuing through the formation of additional phenolic free radicals and coupling at a myriad of sites to form a huge covalent lignin polymer. Figure \(\PageIndex{30}\) shows one example of a larger lignin.

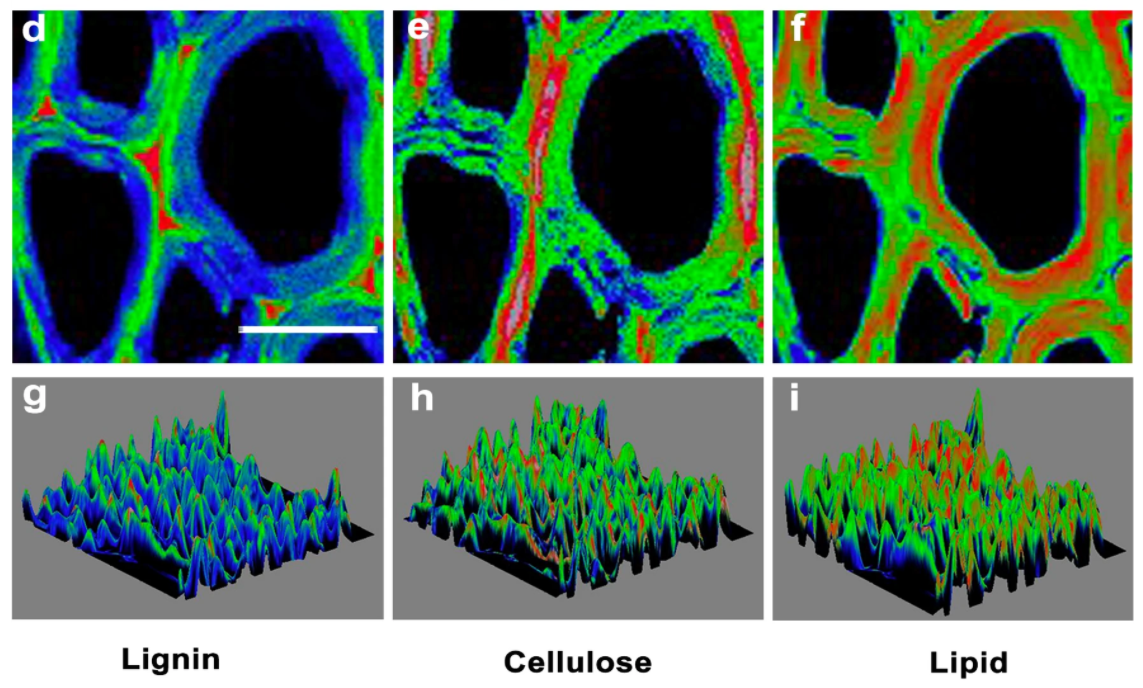
Finally, Figure \(\PageIndex{31}\) shows an image of a poplar tree cell wall, made using surface Raman scattering, showing lignin, cellulose, and lipids in secondary xylem cell walls.
The Extracellular Matrix (ECM) and Basement Membranes
We won't formally discuss cell membranes until Chapter 11, but since anyone reading this book has previously seen biological membranes (including the Gram-negative and positive bilayers discussed above), let's explore a term that most chemistry students, but perhaps not biology students, will find very confusing. That topic is the basement membrane. The basement membrane is encountered often so often, that we will explore its overall structure here even though it is not a lipid bilayer. It fits well here since it is a complex structure consisting of proteins and proteoglycans. It's very amorphous which makes its structure difficult to those hoping for crystal structures or even complex bilayers. It is somewhat similar to the cell wall in functionality. We will offer a cursory explanation. For a great overall introduction, please visit Introduction to Extracellular Matrix and Cell Adhesion in BioLibre texts. Some of the images (when noted) below come from that Cell Biology book chapter.
The extracellular matrix (ECM) is a general term for the large protein and polysaccharide network formed on secretion by some cells in a multicellular organism. They act as connective material to hold cells in a defined space. Cell density can vary greatly between different tissues of an animal, from tightly-packed muscle cells with many direct cell-to-cell contacts to liver tissue, in which some of the cells are only loosely organized, suspended in a web of extracellular matrix, shown in Figure \(\PageIndex{32}\).

The ECM is a generic term encompassing mixtures of polysaccharides and proteins, including collagens, bronectins, laminins, and proteoglycans, all secreted by the cell. The proportions of these components can vary greatly depending on tissue type. Two, quite different, examples of ECM are the basement membrane underlying the epidermis of the skin, a thin, almost two-dimensional layer that helps to organize the skin cells into a nearly-impenetrable barrier to most simple biological insults, and the massive three-dimensional matrix surrounding each chondrocyte in cartilaginous tissue. The ability of the cartilage in your knee to withstand the repeated shock of your footsteps is due to the ECM proteins in which the cells are embedded, not to the cells that are actually rather few in number and sparsely distributed. Although both types of ECM share some components in common, they are distinguishable not just in function or appearance, but in the proportions and identity of the constituent molecules
Figure \(\PageIndex{33}\) shows a general structure of the basement membrane. Think of it as an amorphous polymer mixture (somewhat similar to a polyacrylamide gel).