2.1: The multiple roles of water
- Page ID
- 14920
Search Fundamentals of Biochemistry
“Nothing in the world is as soft and yielding as it,
Yet nothing can better overcome the hard and strong,
For they can neither control nor do away with it.
The soft overcomes the hard,
The yielding overcomes the strong;”
These words come from the Tao Te Ching by Lao Zu. Let’s convert this into a chemical riddle and apply it at the nanoscopic level to biochemistry!
“What it loses it gains,
What it donates it accepts,
It is weak yet strong,
It strengthens yet destroys;”
What is it? The answer (one of many possible) is water! It gains and loses protons, donates and accepts electrons, can be both a weaker or stronger acid/base or oxidizing/reducing agent, and can lead to crystal formation or dissolution, depending on circumstances. Water, at least on our planet, appears necessary for life. We know of no biological life form that exists without it. This molecule has a plethora of properties, which make it unique compared to most other liquids and optimal for the type of life on earth. It has contrasting and oppositional properties. Let’s investigate a few.
Water as a solvent
Solubility is a property that depends on the nature of both solute and solvent. To a first approximation, We tell students in introductory chemistry and biology courses that for a solute to dissolve in a solvent, and form a solution (an example of a homogenous mixture), the sum of noncovalent interactions (intermolecular forces) between solute and solvent must be greater than those among solute molecules and those among solvent molecules.
As students advance in chemistry classes, nuance is added to that general understanding as entropic contributions to solubility must be considered. Entropy is often described as the degree of apparent disorder in the system. Given that description, changes in entropy would appear to favor the soluble state as a solution of the solute in solvent would be more disordered. That simple description must be adjusted to account for the ordered state of solvent (a clathrate) surrounding a solute and of “holes” in the solvent that accommodate larger solute molecules. Enthalpy considerations also must be considered. The description of entropy as a measure of disorder is not precise. Rather it should be described as a measure of the number of microstates of energy or particles available within a system. An entropy increase would arise from an increase in the number of such available microstates, which could correlate with an increase in the disorder of a system.
Students might often consider a molecule as either soluble or insoluble in a given solvent. This notion can be reinforced by simple liquid/liquid partitioning experiments in organic chemistry experiments in which two immiscible solvents (for example water and an ether) are used. Yet diethyl ether is partially soluble in water (1 g/100 mL). Nonpolar molecules with no or few bond dipoles are generally considered insoluble. Students would know that acetic acid, a two-carbon molecule, is soluble in water, but how many carbons are necessary for the molecule to become essentially insoluble? Molecules with a single polar group (-OH, CO2H) and a long alkyl/acyl chain are best described as amphiphilic. Amphiphiles like octanol (C8H17OH) and dodecyl sulfate (CH3(CH2)10CO2H) can form multimolecular aggregates called micelles even as they exist in as a biphasic system, as shown in the following equilibria:
\[\ce{C8H17OH(liq) ↔ C8H17OH(aq) <=> C8H17OH(micelle)}. \nonumber \]
Figure \(\PageIndex{1}\) shows an interactive iCn3D model of a micelle below, which consists of 54 self-associated molecules of dodecylphosphocholine fatty acids. It has almost "complete" separation of polar (on the surface) and nonpolar atoms (buried).
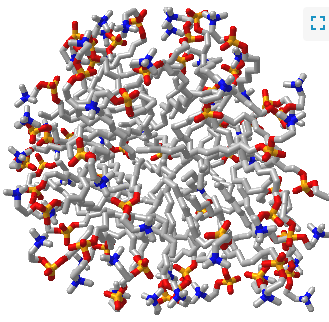
Note the grey lines representing the nonpolar tails are buried from the surrounding water molecules, which form H bonds with the polar head groups.
Without some limited solubility, the following reaction could not occur:
\[\ce{nC8H17OH(aq) ↔1-C8H17OH(micelle).} \nonumber \]
To solve the general problem of the limited solubility of organic molecules in aqueous-based life, biomolecular structures have evolved to “transport” mostly nonpolar molecules like long-chain carboxylic acids (fatty acids) and cholesterol in circulation. The structure of one such fatty acid and cholesterol-containing particle, nascent high-density lipoprotein (HDL), has been determined by small-angle neutron scattering. Figure \(\PageIndex{2}\) shows an interactive iCn3D model of it. The gray sticks represent the nonpolar, acyl tails of the long-chain carboxylic (fatty) acids while the polar red (oxygen) and blue (nitrogen) atoms surrounding the surface are polar groups connected to the tails. The long magenta and dark blue "helices" represent a protein that wraps around the particle and stabilizes it.
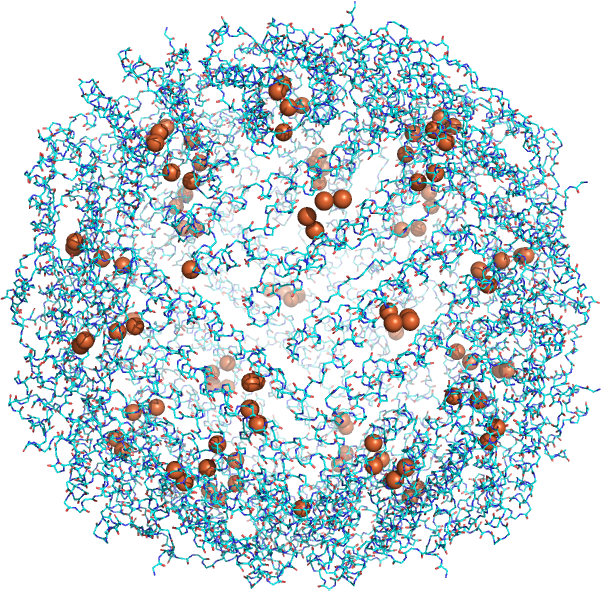
The same ideas can be applied to the solubility of salts. Students will remember general solubility rules (all Gp 1 and Gp 7 salts are soluble) from introductory chemistry. Salts of divalent cations are less soluble as the attractive ion-ion forces within the solid crystal lattice are too strong for the compensatory ion-dipole interactions between the ion and water. Hence salts of Ca2+ and Fe2+ ions such as CaCO3 and FeCO3 are generally insoluble (Ksp values of 1.4 x 10-8 and 3.1 x 10-11, respectively). Insoluble calcium salts (carbonates and silicates) are need for shells of Foraminifera and skeletons of vertebrates. Yet free Ca2+ and Fe2+ ion are found in extracellular and intracellular compartments. Divalent cations like Fe2+ can be toxic at a higher concentration so ways to effectively transport and sequester them have evolved. Figure \(\PageIndex{3}\) shows the structure of human heavy-chain ferritin (4zjk), a protein that forms a hollow shell in which is stored Fe2+ ions (along with counter ions). The model below shows a ferritin with 120 Fe2+ ions (spheres) inside the hollow ferritin sphere.
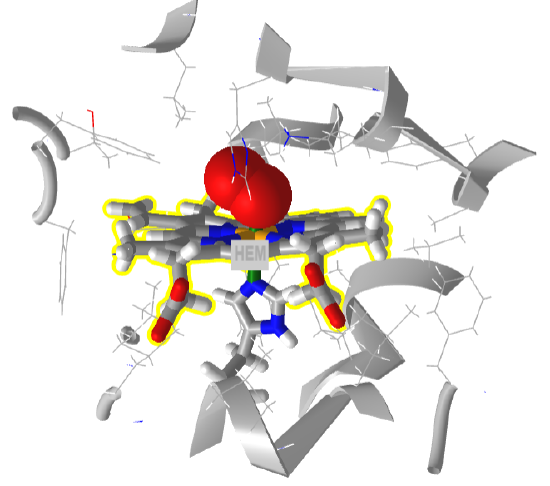
Finally, let’s consider the solubility of gases. The ones that are the most abundant and relevant are O2 and CO2 as they are the reactants and products of oxidative respiration. The gases, although they contain oxygen atoms, are nonpolar and have no net dipole. Hence they are quite insoluble in water. However, they must be soluble enough to allow fish to extract it from water. To solve the solubility problem, evolution has produced proteins like vertebrate hemoglobin that bind oxygen through a transition metal complex containing Fe2+-heme complex (hemoglobin in vertebrates). Some invertebrates use the transition metal Cu ions in hemocyanins for the same purpose. Figure \(\PageIndex{4}\) shows an interactive iCn3D model of dioxygen (red spheres), bound to a planar heme (yellow highlights) which contains an Fe2+ at its center (not shown) at it center in human hemoglobin (6BB5)
Water engages in noncovalent interactions with itself and other molecules. Individual noncovalent interactions are weak but if there are many they can lead to very strong interactions. You've studied noncovalent interactions before, which may have been described as “intermolecular forces”. We prefer the term noncovalent interaction. These include ion-ion, ion-dipole, hydrogen bonds, dipole-dipole, induced dipole-induced dipole, and other variants.
All of these interactions originate in the electrostatic force between two charged objects. There is only one law that describes the forces of attraction, and that’s Coulomb’s Law:
\[F=\dfrac{k Q_{1} Q_{2}}{r^{2}} \nonumber \]
From this force derives all the electrostatic interactions listed above. The magnitude of the attractions for these electrostatic interactions depends on the way charge is distributed in the attracting species. We will explore these in depth in Chapter 2.4.
Water as a reactant: Acids and Bases
H2O, with its sharable lone pairs and slightly positive Hs is both a Brønsted–Lowry base and acid. Its acid-base chemistry hence is among its most important features.
Water, acting as a base, can react with both strong and weak acids. Examples of reactions of a strong acid (\(\ce{HCl}\)) and weak acids (acetic acids and ammonium) with water as a base are shown in Figure \(\PageIndex{5}\).
Likewise, water can act as an acid as demonstrated in Figure \(\PageIndex{6}\).
In the first example, no net changes occur. In the second, a negatively charged deprotonated amine (a stronger base than water) can accept a proton from water, which acts as an acid. All acid/base reactions go predominantly in the direction of stronger acid/strong base to weaker acid/weaker base. Whether water reacts with a strong acid, such as HCl, or a weak one like acetic acid, the strongest acid that can actually exist in an aqueous system is H3O+(aq). This is an example of the leveling effect.
Water as a reactant: nucleophile/electrophile
In the reactions above, we characterized water as a Brønsted–Lowry acid or base. More generically, we could have said water is a Lewis acid (electron pair acceptor) or Lewis base (electron-pair donor). In many reactions, we can also call water a nucleophile (when it shares its lone pair) or an electrophile (when its slightly positive H atoms react with a nucleophile. Here are some examples.
Reaction of water with a transition metal complex.
This reaction below is effectively a nucleophilic substitution reaction in which water displaces ammonia as a ligand as shown in Figure \(\PageIndex{7}\) and the following chemical equation.
\[\ce{[Cu(NH3)4(H2O)2]^{2+} + 4H2O <=> [Cu(H2O)6]^{2+} + 4NH3 } \nonumber \]
Hydration of an alkene
The reaction is catalyzed by the addition of a proton from an acid (like H2SO4) which can be called an electrophilic hydration. Once protonated at the carbon which makes the most stable carbocation, water as a nucleophile attacks the positive carbon to produce the alcohol. These steps are illustrated in Figure \(\PageIndex{8}\).
Nucleophilic substitution at an electrophilic carbonyl
This is a very common reaction. When water is the nucleophile, the reaction is also called a hydrolysis reaction. The reactions in Figure \(\PageIndex{9}\) are shown with OH- as the nucleophile instead of water for simplicity.
Water as a reactant: Oxidizing/Reducing agent
Everyone knows what happens if you throw a piece of solid Na or K into water. An extremely exothermic reaction occurs which releases \(\ce{H2}\) gas which can catch fire and lead to an explosion. The reaction of Na is:
\[\ce{2Na(s) + H2O → 2Na^{+}(aq) + OH^{-} (aq) + H2(g) .} \nonumber \]
The oxidation number of elemental sodium is 0, while Na+ is +1, indicating that the sodium metal has been oxidized by the water which acts as an oxidizing agent.
This reaction occurs with many pure metals, but some that are less reactive (remember the activity series from introductory chemistry?) require acid, a protonated form of water, as shown in the reaction below:
\[\ce{Zn(s) + 2H3O^{+}(aq) ⟶ Zn^{2+} (aq) +2H2O(l) +H2(g)}\nonumber \]
As in acid/base reactions, in a redox reaction, an oxidizing agent and a reducing agent react to form a new oxidizing and reducing agent. Other reactants can oxidize water to form oxygen. Consider fluorine gas for example:
\[\ce{3F2 + 2H2O -> O2 + 4HF}\nonumber \]
F2 is a strong oxidizing agent (as you would surmise from its electronegativity) than O2 so the reaction proceeds vigorously to the right.
Of more biological relevance is the oxidation of water to produce O2 in photosynthesis, a complex series of reactions that is effectively the reverse of combustion:
\[\ce{6CO2 (g) + 6H2O (l) → C6H12O6(s) + 6O2(g).}\nonumber \]
This reaction obviously is endergonic and requires a large input of energy so the reaction proceeds to produce the potent oxidizing agent O2. The special oxygen-evolving complex in photosynthesis is the powerful oxidant that can oxidize H2O to form the weaker oxidizing agent, O2.



