28.1: General Features of Signal Transduction
- Page ID
- 14990
Introduction to Cell Signaling
Cell signaling is at the heart of biology. A cell must know how to respond to chemical signals in its environment. These signals control every aspect of cell life and interactions. A cell must sense when to grow, divide and die. It must sense the presence of foreign and toxic molecules. It must defend itself. The membrane represents the divide between the outside and inside world. Signals must cross that divide and this most often happens without the signaling molecule entering the cell. Just the signal itself is transduced across the membrane. And it doesn't stop there. Internal signaling, also across internal membranes of organelles in eukaryotes, propagates spatially and temporally across the cytoplasm in all cells (prokaryotes, Archaea, and eukaryotes). It's impossible to describe the myriad of processes that occur in cell signaling in a single chapter, but we will do our best to present the common features used across cells.
In addition, signals in complex organisms must be integrated within tissues and organs, between organs (brain and liver for example), and within the entire organisms. Mathematical modeling is critical in understanding the complexity of these interconnected interactions. Consider the flee, fight, or freeze responses. What if you were walking down the street and suddenly saw a tiger approaching you? Most would flee. In that process, the neural, muscular, and metabolic systems must be integrated. The resting state is followed by the activation of the flight/flee response, the maintenance of the fleeing state, and then the return to the resting state. An alternative is the freeze response, which in some situations would be adaptive.
Perhaps the most complex signaling occurs in the great communication networks in the neural and immune systems. Think of it. You can experience a traumatic or emotionally-charged event once and remember it forever. Ordinary events leave less permanent traces. The biochemical changes accompanying long and short-term memory are fascinating
Let's consider general principles for signal transduction across membranes of any cell that must respond to its environment. Typically the agent that signals a cell to respond is a molecular signal. Signaling can also be mediated by pressure (for touch and hearing) or light for vision. The chemical signal binds either to a cell surface receptor or to a cytoplasmic receptor if the signaling agent is hydrophobic and can transit the membrane bilayer.
The logic of signaling
To a first approximation, you could consider a cell as a black box, as shown in Figure \(\PageIndex{1}\), with input (an external molecule for example) and resulting output signals within a cell, such as activation of gene transcription, trafficking of molecules within the cell, chemical reactions, or even export of molecules from the cell. There could be one or more signals, and one or more outputs. The inputs might arrive gradually and reach a threshold before triggering an output, or the input might be abrupt leading to an immediate output. This Figure \(\PageIndex{1}\):
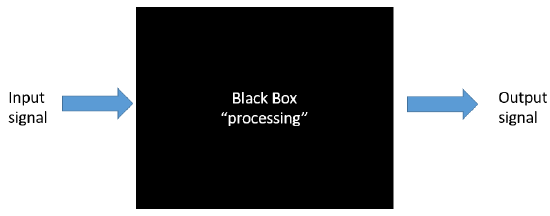
Note the use of terminology taken from the world of electronics. Figure \(\PageIndex{2}\) shows the similarity in the complexity of an electronic wiring diagram (left) to the interconnected metabolic pathways of a cell (right).
The analogy is much greater than you might think. Cell signaling researchers have adopted the language and symbols of electronics as they consider how two inputs could lead to different output signals. Figure \(\PageIndex{3}\) shows a truth table with different Boolean operator logic gates and symbols used by electrical engineers that also apply to signals to cells and their outputs. Two input signals, A and B, arrive at different logic gates named AND, NOR, OR, XOR AND, and NAND. 0 indicates no signal (either input or output) while 1 indicates a signal (either input or output).
Each of these logic gates leads to different outputs:
- AND gates require both A and B input signals for an output;
- OR gates require either A or B or both for an output signal;
- NOR gates require neither A or B for an output signal;
- NAND gates normally have an output signal unless both inputs A and B are present;
- XOR (Exclusionary OR) gates give a signal if the two inputs A and B differ.
An example of an analogous biological AND gate is N-WASP protein [a homolog to the Wiskott-Aldrich syndrome protein (WASP)]. This protein regulates actin polymerization and binds multiple signals. Stimulation with two proteins, Cdc42 and phosphatidylinositol (4,5)-bisphosphate (PIP2), activates polymerization.
Researchers are designing protein logic gates by creating a series of heterodimeric proteins. Consider the following heterodimers: A:A', B:B', and C:C', where the second member in each pair is different from the first and bound reversibly through noncovalent interactions (indicated by the colon :) Other versions could exist, such as A:C'. An AND gate for the formation of an A:C' dimer would be made by making the covalent, single molecules A'-B and B'-C. The A:C' dimer could form only in the presence of both A'-B and B'-C. This is illustrated in Figure \(\PageIndex{4}\).
Signals and their responses must occur at the right time under the right conditions and for the right duration. Under opposing sets of conditions (for example well feed and starving), opposing signaling pathways, mediated by different signaling molecules, must be mutually integrated and regulated so one is turned on and one shut off. Methods must be in place to terminate signal effects.
Given the complexity of signaling pathways, it's hard to know how to present the material in a single chapter. Binding initiates and mediates almost all biological events. That binding must also be specific to avoid off-target effects. If you were to predict what biomolecule could confer specificity in the binding of a signal and allow changes from the unbound to bound state, you would certainly pick proteins. Protein:ligand binding is key to understanding biosignaling. Other types of biomolecules such as lipids and nucleic acid are involved and are of clear importance, but they are typically involved downstream from the initial protein:signal binding event. Hence we will focus mostly on signaling proteins and conformational/activity changes that occur on signal binding.
To simplify protein involvement in signaling, we can assume signaling proteins have an active and inactive state. The states are interconvertible (reversible). Let's assume the signaling protein is an enzyme. The activity of the enzyme depends on many factors as described in Figure (\PageIndex{5}\) below. The green color represents the active enzyme, while red indicated inactive.
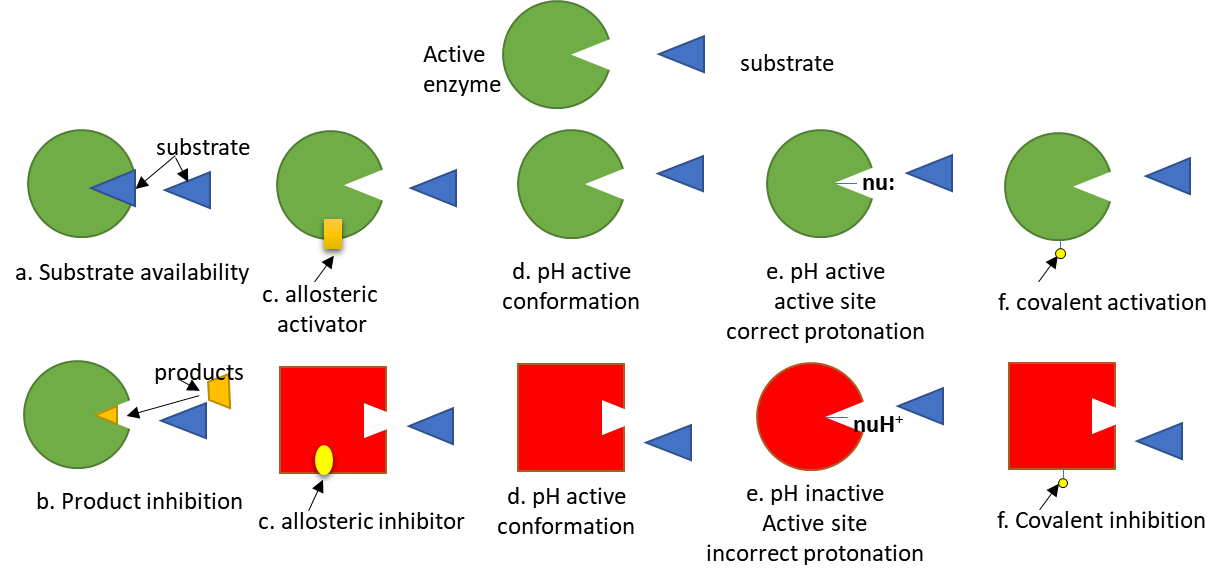
Of course, proteins that are not enzymes (for example transcription factors) can be regulated similarly.
In addition, the amount (and localization as well) of a signaling protein can regulate the activity of the protein, as illustrated in Figure (\PageIndex{6}\) below.
Each signaling protein can be considered to be a node in a larger pathway consisting of interconnected nodes. This chapter on cell signaling hence can be viewed as a capstone to Unit 1, which explores the structure and function of biomolecules and as a prelude to the study of whole metabolic pathways.
Second Messengers and Signal Translocation
The chemical species that trigger signaling typically binds to a target protein transmembrane receptor on the surface of a cell but does not itself enter the cell. Just the signal enters the cell. If you were to hypothesize how that might happen, you would predict two mechanisms:
- an integral transmembrane receptor undergoes a conformational change on binding that propagates to its cytoplasmic domain, which can then interact either with other cytoplasmic proteins or cytoplasmic domains of other membrane proteins, transmitting the symbol into the cytoplasm;
- the bound conformation of the receptor has enzyme activity and can catalyze chemical reactions on the luminal side of the membrane. This could include the chemical modification of proteins or the synthesis of new small molecules from cell metabolites. These new small molecules are called second messengers. They could be formed in the membrane bilayer or the cytoplasm.
There are several diverse types of second messengers. Two common ones are cyclic derivatives of small nucleotides, including cyclic AMP (cAMP) derived from ATP and cyclic GMP (cGMP) derived from GTP. Ca2+ ions are usually found in low concentrations in the cytoplasm as they are pumped into internal organelles such as the endoplasmic reticulum and mitochondrion. Signaling processes can release Ca2+ ions in waves within the cell. Membrane lipids are also processed to form second messengers. Membrane phospholipids can be cleaved by cell signaling-activated lipases to form free arachidonic acid, sphingosine, diacylglycerol, and inositol-trisphosphate, which can act as second messengers. Redox signaling in the cell can also occur through hydroperoxides acting as second messengers.
What if the response of the cell requires gene transcription? Somehow the signal has to translocate from the cell membrane through the cytoplasm through the nuclear membrane into the nucleus. Hence a series of translocations of multiple downstream signaling events must occur.
We have already seen how newly synthesized proteins have signal sequences that target them to specific cellular locations like the cell membrane, mitochondria, nucleus (nuclear localization sequences - NLS and the small GTP binding protein RAN), or for export. Proteins involved in signaling also can move throughout the cell as part of the signaling process. Likewise, we have seen how cytoplasmic proteins can be targeted to membranes by attachment of fatty acids or isoprenoids.
Post-translational modification of signaling proteins
Nature has chosen the post-translational modifications (PTM) of proteins as a ubiquitous way to alter the signaling states of proteins. As we have seen previously, PTMs can alter protein conformation. They can also present new binding interfaces that allow interaction with other signaling proteins. The main (but not the only) PTM used for signaling is the reversible phosphorylation of the OH-containing amino acid side chains (Tyr, Ser, and Thr) as well as histidine (mostly in prokaryotes), so we will focus on them. Enzymes that catalyse the phosphorylation of proteins are called protein kinases. Reversibility is important since if phosphorylation of a target protein is associated with a specific signaling change (either activation or inhibition), then dephosphorylation can easily reverse the signaling event. Enzymes that dephosphorylate phosphoproteins are called protein phosphatases. Figure \(\PageIndex{7}\) shows the generic reaction of protein kinases and phosphatases.
There appear to be 518 protein kinases and 199 protein phosphatases encoded in the human genome. Why so many? If just one protein kinase existed, one mutation in it would be disastrous. In addition, the large number of kinases and phosphatase allows for great control in the specificity of these enzymes for their target protein substrates.
Kinases
Kinases are a class of enzymes that use ATP to phosphorylate molecules within the cell.
The names given to kinases show the substrate which is phosphorylated by the enzyme. For example:
- hexokinase - an enzyme that uses ATP to phosphorylate hexoses.
- protein kinase - enzymes that use ATP to phosphorylate proteins within the cell. (Note: Hexokinase is a protein, but is not a protein kinase).
- phosphorylase kinase: an enzyme that uses ATP to phosphorylate the protein phosphorylase within the cell
If a protein is phosphorylated by a kinase, the phosphate group must eventually be removed by a phosphatase through hydrolysis. If it wasn't, the phosphorylated protein would be in a constant state of either being activated or inhibited. Kinases and phosphatases regulate all aspects of cellular function. About 1-2% of the entire genome encodes kinases and phosphatases.
Kinases can be classified in many ways. One is substrate specificity: Eukaryotes have different kinases that phosphorylate serine/threonine or tyrosine side chains. Prokaryotes also have His and Asp kinases, but these are unrelated structurally to the eukaryotic kinases. There are 11 structurally different families of eukaryotic kinases, which all fold to a similar active site with an activation loop and catalytic loop between which substrates (ATP and the OH-containing side chain) bind. Simple, single-cell eukaryotic cells (like yeast) have predominantly cytoplasmic Ser/Thr kinases, while more complex eukaryotic cells (like human cells) have many Tyr kinases. These include the membrane-receptor Tyr kinases and the cytoplasmic Src kinases.
Manning et al. have analyzed the entire human genome (DNA and transcripts) and have identified 518 different protein kinases, which cluster into 7 main families as shown in Table \(\PageIndex{1}\): below. Family membership was determined by sequence comparisons of catalytic domains. The entire repertoire of kinases in the genome is called the kinome. Alterations in 218 of these appear to be associated with human diseases.
| Name | Description |
|---|---|
| AGC | Contain PKA, PKG, and PKC families |
| CAMK | Ca2+/CAM-dependent PK |
| CKI | Casein kinase 1 |
| CMGC | Contain CDK, MAPK,GSK3, CLK families |
| STE | homologs of yeast sterile 7, 11, 20 kinases; MAP Kinase |
| PTK | Protein tyrosine kinase |
| PTKL | Protein tyrosine kinase-like |
| RGC | Receptor guanylate kinase |
Table \(\PageIndex{1}\): The human kinome
Phosphatases
There are three main families of phosphatases, the phospho-Tyr phosphatases (PTP), the phospho-Ser/Thr phosphatases, and those that cleave both. Of all phosphorylation sites, most (86%) are on Ser, 12% involve Thr, and about 2% on Tyr. They can also be categorized by their molecular sizes, inhibitors, divalent cation requirements, etc. In contrast to kinases which differ in the structure of their catalytic domains, many phosphatases (PPs below) gain specificity by binding protein cofactors which facilitate translocation and binding to specific phosphoproteins. The active phosphatase hence often consists of a complex of the phosphatase catalytic subunit and a regulatory subunit. Regulatory subunits for Tyr phosphatases may contain a SH2 domain allowing binding of the binary complex to autophosphorylated membrane receptor Tyr kinases.
Given this background, we can now start to explore signal transduction in a methodical way. There are two major ways to organize our discussions:
- start from the binding of the molecular signal at the cell membrane and trace the signaling events inward into the cell, potentially all the way to the nucleus and gene expression
- describe recurring motifs found in most pathways.
We will use a combination of both, but it makes sense to start with the signaling proteins at the cell membrane. Next, we will focus on second messengers. We'll follow that with detailed explorations of kinases and phosphatases and specific signaling pathways.



