1.11: Understanding the Importance of Buffers in Biological Systems
- Page ID
- 18914
Learning Objectives
You should be aware that buffers play a critical role in almost all biochemical systems. Biochemical experiments routinely require a buffer. In this laboratory you will cover the basics of buffer preparation and test the buffering capacity of the resulting solution. This buffer will be used in the enzyme kinetics (acetylcholinesterase) lab later in the term.
11.2 Introduction
Most biochemical reactions either produce or consume hydrogen ions. Take, for example, the reaction catalyzed by acetylcholinesterase. This enzyme rapidly destroys the neurotransmitter acetylcholine (Ach) after it has delivered its signal across synapses such as the neuromuscular junction. The acetylcholinesterase reaction hydrolyzes acetylcholine, to choline, acetate and a hydrogen ion. Note the hydrogen ion liberated by this reaction.
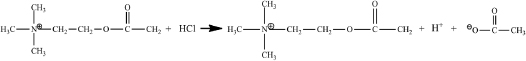
Typically, during nerve signaling the synaptic [Ach] will increase from zero to the millimolar concentration range. Destroying this acetylcholine will release millimolar quantities of hydrogen ions. If uncontrolled, this simple reaction would decrease the pH from about 7 to below 6. This pH change is far beyond anything that human cells can tolerate. Similar wide swings in pH can arise from almost every metabolic process. So, pH control is necessary for life; this control is provided by buffers.
11.3 Buffer Preparation
Buffers are an aqueous solutions of weak acids or bases that minimize a pH change. Because these acids/bases are “weak,” they establish an equilibrium in solution
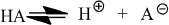
that can be described with the equation,

Rearranging Equation 1:

Equation 2 shows that the hydrogen ion concentration depends on the conjugate acid concentration [HA] and the conjugate base concentration [A-] as well as the equilibrium constant Ka. That is, the [H+] is under control.
Because pH is commonly measured instead of [H+], Equation 3 is most often presented in a modified form (the Henderson-Hasselbalch Equation):
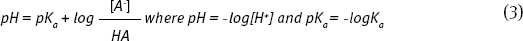
This equation emphasizes that pH changes as the ratio of concentrations of A- to HA changes. So, adjusting the concentration of [HA] and [A-] will set the pH.
For example:
– In pure water, the pH is 7. Adding HCl (a strong acid) to 0.001 M will increase the [H+] to about 0.001 M. The pH will change to about 3, a difference of four pH units.
– In a buffer solution, the same addition of HCl will cause a shift in the buffer equilibrium, rather than a drastic pH change. For example, let [A-] = [HA] = 0.1 M before adding HCl.
- - Adding 0.001 M HCl will convert 0.001 M A- to 0.001 M HA.
- - The buffer concentrations will change, [A-] to 0.099 M and [HA] to 0.101 M.
- - The
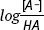 will change from
will change from  to
to 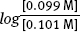 . According to the Henderson-Hasselbalch Equation, the pH will decrease by 0.009 units.
. According to the Henderson-Hasselbalch Equation, the pH will decrease by 0.009 units.
– Instead of reacting with water and changing the pH by four units, the HCl reacts with the buffer and only changes the pH by 0.009 units.
Four important generalizations about buffers
- A buffer is composed of an equilibrium mixture of a weak acid (HA) and its conjugate weak base (A-).
- The higher the buffer concentration, the greater the pH control.
- No matter what the buffer concentration, maximum pH control is reached when [HA] = [A-]. At this point, the Henderson-Hasselbalch Equation gives pH = pKa + 0. So, the maximum pH control occurs when the pH is numerically equal to the pKa.
- It is good practice to choose a weak acid whose pKa is close to the pH you are targeting. Typically, weak acids are effective buffers at pHs within one unit of their pKa.
Procedures
Reagents and equipment needs are calculated per six student teams. There is ~20% excess included.
Equipment/glassware needed
- six 100 ml beakers
- six stir bars
- six stir plates
- six 100 ml graduated cylinders
- six pH meters of same kind
- small box of plastic Pasteur pipets
Solutions needed
- 250 ml 0.5 M HCl
- solid tris HCl
- solid tris base
- pH 7.0 standard buffer
- pH 4.0 standard buffer
I. Using the conjugate acid, trisHCl, and the conjugate base, tris, prepare one hundred milliliters of a 0.2 M buffer at pH = 8.0. (Tris has a pKa = 8.1.)
- Using the Henderson-Hasselbalch equation, determine the concentrations of both [A-] = tris = x and [HA] = trisHCL = (0.2 - x) [A-].
- Calculate masses needed.
- Add the masses to a 100.00 ml volumetric flask and fill to the mark with “Millipore” water.
- Transfer the stock buffer to a bottle, appropriately labeled with the contents (0.2 M tris buffer), the pH, your name(s) and date.
II. Using a pH meter, determine buffer pH and buffering capacity.
A pH meter is a common piece of laboratory equipment that requires some care in use. The glass bulb on the end of the electrode is fragile and easily broken. Always rinse the electrode with distilled water when moving from one solution to another. The pH meter should be set in standby mode when the electrode is out of solution.
A pH meter offers a relative measure of pH and, therefore, must be standardized. Typically, two standard buffers are used. A first buffer (commonly, pH=7.0) is used to make major adjustments; then, a second buffer (pH=4.0) is used to make fine adjustments. The pH meter will have two different dials - one for major adjustments and one for fine adjustments.
- Measure the pH of your buffer solution.
- Prepare 32 ml of a 1:4 dilution of your buffer. (Graduated cylinders may be used for the volume measurements.) Measure the pH of this dilution.
- Add 0.5 M HCl dropwise to drive the pH down below 6.
- Titrate the buffer solution back to pH 11 with 0.1 M NaOH. Record about twenty points to graph your titration curve later. Your burette readings should be taken to the nearest 0.05 ml.
- Prepare 32 ml of a 1:40 dilution of your buffer. (In addition to a graduated cylinder, use a pipette for this dilution.) Measure the pH of this dilution.
- Add 0.5 M HCl dropwise to drive the pH down below 6.
- Titrate the buffer solution back to pH 11 with 0.1 M NaOH. Record about twenty points to graph your titration curve later. Your burette readings should be taken to the nearest 0.05 ml.
- Store your buffer at 4 °C for use with the acetylcholinesterase kinetics laboratory later in the semester.
III. Graph the titrations.
- Using a computer graphing program, plot pH versus mmol of OH- for both titrations. Note the impact of tris on the pH change.
- Qualitatively, buffering capacity can be defined as the amount of strong acid/base that can be added to a buffer solution before causing a significant pH change. Buffering capacity can be quantified by taking the inverse of the instantaneous slope of pH vs. OH- amount. A quick, empirical approach to measuring the buffering capacity is to note the amount of NaOH needed to change the pH by one unit in the middle of the buffering region. Using this last method, determine the buffering capacity for your tris solution from each titration.
Notes to the instructor
The tris/trisHCl buffer was chosen to accommodate the acetylcholinesterase enzyme kinetics lab later in the term. Any buffer system may be substituted. From a pedagogical perspective, a follow-up lab that uses the buffer to study a biochemical reaction is appropriate.
Prelab for the Buffer Lab
1. (2 pts.) Describe the reaction catalyzed by acetylcholine esterase!
2. (2 pts.) Define/describe buffer.
3. (6 pts.) During the lab you will prepare 100 ml of Tris buffer at pH=8.0 using the conjugate acid, trisHCl, and the conjugate base, Tris, (Tris has a pKa =8.1.).
a. Using the Henderson-Hasselbalch equation, determine the concentrations of both [A-] = tris = x and [HA] = trisHCl = (0.2 - x).
b. Calculate masses as needed.
Buffer Lab Report
Outline and Point Distributions
Introduction:
1. Write several sentences defining the goal/purpose of this experiment (3 pts.).
Data:
- Show the calculations you used to prepare the 0.2 M tris buffer (3 pts.).
- Show the calculations you used to prepare the 1:4 and 1:40 diluted tris buffer (3 pts.)
- Report the pH for all three buffers (3 pts.).
Results:
- Graph both titrations using a computer graphing program (3 pts./graph).
- Show how you determined the pKa and the buffering capacity on your graph for each titration (4 pts.).
- Report the buffering capacity and pKa as determined for each titration (4 pts.).
Analysis:
- Suggest some possibilities as to why your buffer pH might not be exactly 8.0 (3 pts.).
- Does the center point of the tris buffering region (“best buffering”) match the pKa? Briefly explain (3 pts.).
- Does the buffering capacity change in a predictable way? Briefly explain (3 pts.).
Questions/Problems:
- Include the solutions to the Buffer Problems handout (15 pts.).
Buffer Problem Set
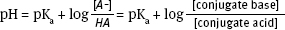
1. Calculate the pH of a one liter solution containing 0.15 mol benzoic acid and 0.25 mol sodium benzoate. The pKa for benzoic acid is 4.2.
The next set of problems illustrates the two common methods to prepare a buffer.
2. Add a known amount of conjugate acid to a known amount of conjugate base: What is the pH of 0.5 l of a buffer prepared by mixing 8.6 g of lactic acid (90 g/mol) with 7.8 g of sodium lactate (112 g/mol)? The pKa for lactic acid is 3.86.
3. Calculate the mass of benzoic acid and sodium benzoate (in grams) needed to prepare 250 ml of a 0.1 M buffer at pH = 4.1. (The buffer concentration is defined as the sum of the conjugate acid concentration plus the conjugate base concentration.) The pKa of benzoic acid is 4.2.
4. Start with either the conjugate acid or base and add a strong base or strong acid (conjugate acid plus strong base forms the conjugate base and water; conjugate base plus strong acid forms the conjugate acid): What is the pH of 0.5 l of a 0.1 M acetic acid solution to which 0.73 g of NaOH are added? The pKa of acetic acid is 4.76.
5. You are asked to prepare 1.2 l of a 0.05 M tris buffer at pH= 7.8. You start with the conjugate base form of tris (121 g/mol). How many grams of tris must you weigh out? How many ml of 6 M HCl (a strong acid) must you add to reach pH=7.8? The pKa for tris is 8.1.


